THORIUM

Overview
Thorium is a member of the actinide family. The actinide elements are located in Row 7 of the periodic table. They have atomic numbers between 90 and 103. The periodic table is a chart that shows how chemical elements are related to one another. The actinide series is named for element 89, actinium, which is sometimes included in the actinide family.
Thorium was discovered in 1828 by Swedish chemist Jons Jakob Berzelius (1779-1848). At the time, Berzelius did not realize that thorium was radioactive. That was discovered 70 years later, in 1898, by Polish-French physicist Marie Curie (1867-1934) and English chemist Gerhard C. Schmidt (1864-1949).
Thorium is a relatively common element with few commercial applications. There is some hope that it can someday be used in nuclear power plants, in which nuclear reactions are used to generate electricity.
SYMBOL
Th
ATOMIC NUMBER
90
ATOMIC MASS
232.0381
FAMILY
Actinide
PRONUNCIATION
THOR-ee-um
Discovery and naming
In 1815, Berzelius was studying a new mineral found in the Falun district of Sweden. From his analysis, he concluded that he had found a new element. He named the element thorium, in honor of the Scandinavian god Thor.
Ten years later, Berzelius announced that he had made an error. The substance he had found was not a new element, but the compound yttrium phosphate (YPO 4 ).
Shortly thereafter, Berzelius again reported that he had found a new element. This time he was correct. He chose to retain thorium as the name for this element.
At the time Berzelius made his discovery, the concept of radioactivity was unknown. Radioactivity refers to the process by which an element spontaneously breaks down and gives off radiation. In that process, the element often changes into a new element. One of the first scientists to study radioactivity was Curie. She and Schmidt announced at almost the same time in 1898 that Berzelius' thorium was radioactive.
Physical properties
Thorium is a silvery white, soft, metal, somewhat similar to lead . It can be hammered, rolled, bent, cut, shaped, and welded rather easily. Its general physical properties are somewhat similar to those of lead. It has a melting point of about 1,800°C (3,300°F) and a boiling point of about 4,500°C (8,100°F). The density of thorium is about 11.7 grams per cubic centimeter.
Chemical properties
Thorium is soluble in acids and reacts slowly with oxygen at room temperature. At higher temperatures, it reacts with oxygen more rapidly, forming thorium dioxide (ThO 2 ).
Occurrence in nature
Thorium is a relatively abundant element in the Earth's crust. Scientists estimate that the crust contains about 15 parts per million of the element. That fact is important from a commercial standpoint. It means that thorium is much more abundant than another important radioactive element, uranium . Uranium is used in nuclear reactors to generate electricity and in making nuclear weapons (atomic bombs). Scientists believe thorium can replace uranium for these purposes. With more thorium than uranium available, it would be cheaper to make electricity with thorium than uranium.
The most common ores of thorium are thorite and monazite. Monazite is a relatively common form of beach sand. It can be found, among other places, on the beaches of Florida. This sand may contain up to 10 percent thorium.
Thorium in place of uranium?
U ranium is one of the most important elements in the world today. Why? One of its isotopes undergoes nuclear fission. Nuclear fission occurs when neutrons collide with the nucleus of a uranium atom. When that happens, the uranium nucleus splits apart. Enormous amounts of energy are released. That energy can be used for mass destruction in the form of atomic bombs, or used for peaceful energy production in nuclear power plants.
But there are two problems with using uranium for nuclear fission. First, of uranium's three isotopes (uranium-234, uranium-235, and uranium-238), only one—uranium-235—undergoes fission. The second problem is that this isotope of uranium is quite rare. Out of every 1,000 atoms of uranium, only seven are uranium-235. Tons of uranium ore must be processed and enriched to make tiny amounts of this critical isotope. It is difficult and extremely expensive.
Scientists know that another isotope of uranium, uranium-233, will also undergo fission. The problem is that uranium-233 does not occur in nature. So how can it be used to make atomic weapons or nuclear power?
The trick is to start with an isotope of thorium, thorium-232. Thorium-232 has a very long half life of 14 billion years. If thorium-232 is bombarded with neutrons, it goes through a series of nuclear changes, first to thorium-233, then to protactinium-233, and finally to uranium-233. The whole process only takes about a month. At the end of the month, a supply of uranium-233 has been produced. This isotope of uranium has a fairly long half life, about 163,000 years. So once it has been made, it stays around for a long time. It can then be used for nuclear fission.
Scientists would like to find a way to use this process to make uranium-233 economically. Thorium is much more abundant than uranium. It would be far cheaper to make nuclear bombs and nuclear power plants with thorium than with uranium.
Unfortunately, no one has figured how to make the process work on a large scale. One nuclear reactor using thorium was built near Platteville, Colorado, in 1979. However, a number of economic and technical problems developed. After only ten years of operation, the plant was shut down. The promise of thorium fission plants has yet to become reality.
There is some hope that thorium can someday be used in nuclear power plants, where nuclear reactions are used to generate electricity.
Isotopes
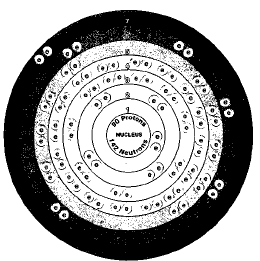
More than two dozen isotopes of thorium are known. All are radioactive. The isotope with the longest half life is thorium-232. Its half life is about 14 billion years. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
The half life of a radioactive element is the time it takes for half of a sample of the element to break down. After one half life (14 billion years), only 5 grams of a ten-gram sample of thorium-232 would be left. The remaining 5 grams would have broken down to form a new isotope.
Extraction
The thorium in monazite, thorite, or other minerals is first converted to
thorium dioxide (ThO
2
). This thorium dioxide is then heated with calcium to get the free
element:
Uses and compounds
Thorium and its compounds have relatively few uses. The most important thorium compound commercially is thorium dioxide. This compound has the highest melting point of any oxide, about 3,300°C (6,000°F). It is used in high-temperature ceramics. A ceramic is a material made from earthy materials, such as sand or clay. Bricks, tiles, cement, and porcelain are examples of ceramics. Thorium dioxide is also used in the manufacture of specialty glass and as a catalyst. A catalyst is a substance used to speed up or slow down a chemical reaction without undergoing any change itself.
The one device in which most people are likely to have seen thorium dioxide is in portable gas lanterns. These lanterns contain a gauzy material called a mantle. Gas passing through the mantle is ignited to produce a very hot, bright white flame. That flame provides the light in the lantern. The mantle in most lanterns was once made of thorium dioxide because it can get very hot without melting.
The thorium dioxide in a gas mantle is radioactive. But it is of no danger to people because the amount used is so small. Still, gas mantles in the United States are no longer made with thorium. Safer substitutes have been found.


Another thorium compound, thorium fluoride (ThF 4 ), is used in carbon arc lamps for movie projectors and searchlights. A carbon arc lamp contains a piece of carbon (charcoal) to which other substances (such as ThF 4 ) have been added. When an electric current is passed through the carbon, it gives off a bright white light. The presence of thorium fluoride makes this light even brighter.
Health effects
As with all radioactive materials, thorium is dangerous to the health of humans and other animals. It must be handled with great caution. Living cells that absorb radiation are damaged or killed. Inhaling a radioactive element is especially dangerous because it exposes fragile internal tissues.
it also should have to include radioactivity of thorium