LEAD

Overview
Lead is the heaviest member of the carbon family. The carbon family consists of the five elements in Group 14 (IVA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. Although a member of the carbon family, lead looks and behaves very differently from carbon.
Lead is one of only a few elements known to ancient peoples. One of the oldest examples of lead is a small statue found in Egypt. It was made during the First Dynasty, in about 3400 B.C. Mention of lead and lead objects can also be found in very old writing from India. And the Bible mentions lead in a number of passages.
SYMBOL
Pb
ATOMIC NUMBER
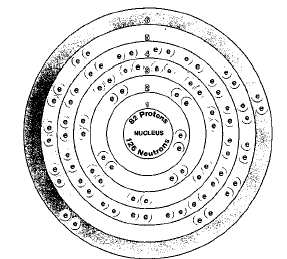
82
ATOMIC MASS
207.2
FAMILY
Group 14 (IVA)
Carbon
PRONUNCIATION
LED
Throughout history, Lead has been used to make water and sewer pipes; roofing; cable coverings; type metal and other alloys; paints; wrappings for food, tobacco, and other products; and as an additive in gasoline. Since the 1960s, however, there has been a growing concern about the health effects of lead. For instance, scientists have found that lead can cause mental and physical problems in growing children. As a result, many common lead products are now being phased out.
Discovery and naming
Lead has been around for thousands of years. It is impossible to say when humans first discovered the element. It does not occur as an element in the earth very often. But one of its ores, lead sulfide (PbS), is fairly common. It is not difficult to obtain pure lead metal from lead sulfide. Humans probably discovered methods for doing so thousands of years ago.
By Roman times, lead metal was widely used. The far-reaching system that brought water to Rome contained many lead pipes. Sheets of lead were used as writing tablets and some Roman coins were also made of lead. Perhaps of greatest interest was the use of lead in making pots and pans. Modern scientists believe many Romans may have become ill and died because of this practice. Cooking liquids in lead utensils tends to make the lead dissolve. It got into the food being cooked. People who ate those foods got more and more lead into their bodies. Eventually, the effects of lead poisoning must have begun to appear.
Of course, the Romans had little understanding of the connection between lead and disease. They probably never realized that they were poisoning themselves by using lead pots and pans.
No one is quite sure how lead got its name. The word has been traced to manuscripts that date to before the 12th century. Romans called the metal plumbum. It is from this name that the element's chemical symbol comes: Pb. Compounds of lead are sometimes called by this old name, such as plumbous chloride.
Physical properties
Lead is a heavy, soft, gray solid. It is both ductile and malleable. Ductile means capable of being drawn into thin wires. Malleable means capable of being hammered into thin sheets. It has a shiny surface when first cut, but it slowly tarnishes (rusts) and becomes dull. Lead is easily worked. "Working" a metal means bending, cutting, shaping, pulling, and otherwise changing the shape of the metal.
The melting point of lead is 327.4°C (621.3°F), and its boiling point is 1,750 to 1,755°C (3,180 to 3,190°F). Its density is 11.34 grams per cubic centimeter. Lead does not conduct an electric current, sound, or vibrations very well.
Chemical properties
Lead is a moderately active metal. It dissolves slowly in water and in most cold acids. It reacts more rapidly with hot acids. It does not react with oxygen in the air readily and does not burn.
Occurrence in nature
The abundance of lead in the Earth's crust is estimated to be between 13 and 20 parts per million. It ranks in the upper third among the elements in terms of its abundance.
Lead rarely occurs as a pure element in the earth. Its most common ore is galena, or lead sulfide (PbS). Other ores of Lead are anglesite, or lead sulfate (PbSO 4 ); cerussite, or lead carbonate (PbCO 3 ); and mimetite (PbCL 2 ○ Pb 3 (AsO 4 ) 2 ).
The largest producers of lead ore in the world are Australia, China, the United States, Peru, Canada, Mexico, and Sweden. In the United States, more than 93 percent of all the lead produced comes from Missouri. Other lead-producing states are Montana, Colorado, Idaho, Illinois, New York, and Tennessee. In 1996, 426,000 metric tons of lead were produced in the United States.
Isotopes
Four naturally occurring isotopes of lead occur. They are lead-204, lead-206, lead-207, and lead-208. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
About sixteen radioactive isotopes of lead are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
Romans routinely ate food cooked in lead pots and pans. The connection between lead and disease was not known then, so many people became ill and died of lead poisoning.
One radioactive isotope of lead, lead-210, is sometimes used in medicine. This isotope gives off radiation that can kill cancer cells. It is also used to treat non-cancerous eye disorders.

Extraction
Lead is obtained from its ores by a method used with many metals. First,
the ore is roasted (heated in air). Roasting, also called smelting,
converts the ore to a compound of lead and oxygen, lead oxide (PbO
2
). Lead oxide is then heated with charcoal (pure carbon). The carbon takes
oxygen away from the lead oxide. It leaves pure lead behind:
Lead obtained in this way is not very pure. It can be purified electrolytically. Electrolytic refining involves passing an electric current through a compound. Very pure lead is collected at one side of the container in which the reaction is carried out.
A major source of lead is recycled car batteries.
Lead is also recovered in recycling programs. Recycling is the process by which a material is retrieved from a product that is no longer used. For example, old car batteries were once just thrown away. Now they are sent to recycling plants where lead can be extracted and used over and over again. It is not necessary to get all the lead that industry needs from new sources, such as ores.
Uses
The lead industry is undergoing dramatic change. Many products once made with lead no longer use the element. The purpose of this change is to reduce the amount of lead that gets into the environment. Examples of such products include ammunition, such as shot and bullets; sheet lead used in building construction; solder; water and sewer pipes; ball bearings; radiation shielding; and gasoline. These changes are possible because manufacturers are finding safer elements to use in place of lead.
The price of a gallon of gas
F or many years, lead was regarded as a miracle chemical by the automotive industry. The power to run a car comes from the burning of gasoline in the engine. However, burning gasoline is not a simple process. Many things happen inside an engine when gasoline burns in the carburetor.
For example, an engine can "knock" if the gasoline does not burn properly. "Knocking" is a "bang-bang" sound from the engine. It occurs when low-grade gasoline is used.
One way to prevent knocking is to use high-grade gasoline. Another way is to add chemicals to the gasoline. The best gasoline additive discovered was a compound called tetraethyl lead (Pb(C 2 H 5 ) 4 ). Tetraethyl lead was usually called "lead" by the automotive industry, the consumer, and advertisers. When someone bought "leaded" gasoline, it contained not lead metal, but tetraethyl lead.
Leaded gasoline was a great discovery. It could be made fairly cheaply and it prevented car engines from knocking. No wonder people thought it was a miracle chemical.
What people didn't realize was that tetraethyl lead breaks down
in a car engine because of the high temperature at which engines
operate. When tetraethyl lead breaks down, elemental lead (Pb) is
formed:
The result—with millions of cars being driven every day—was more and more lead getting into the air. And more and more people inhaled that lead. Eventually, doctors began to see more people with leadrelated diseases.
The federal government finally decided that tetraethyl lead was too dangerous to use in gasoline. By 1990, the use of this compound had been banned by all governments in North America.
Other uses of lead have not declined. The best example is lead storage batteries. A lead storage battery is a device for converting chemical energy into electrical energy. Almost every car and truck has at least one lead storage battery. But no satisfactory substitute for it has been found. About 87 percent of all lead produced in the United States now goes to the manufacture of lead storage batteries. In addition to cars and trucks, these batteries are used for communication networks and emergency power supplies in hospitals, and in forklifts, airline ground equipment, and mining vehicles.
Compounds
A small percentage of lead is used to make lead compounds. Although the amount of lead is small, the variety of uses for these compounds is large. Some examples of important lead compounds are:
lead acetate (Pb(C 2 H 3 O 2 ) 2 ): insecticides; waterproofing; varnishes; dyeing of cloth; production of gold; hair dye
lead antimonate (Pb 3 (SbO 4 ) 2 ): staining of glass, porcelain and other ceramics
lead azide (Pb(N 3 ) 2 ): used as a "primer" for high explosives
lead chromate ("chrome yellow"; PbCrO 4 ): industrial paints (use restricted by law)
lead fluoride (PbF 2 ): used to make lasers; specialized optical glasses
lead iodide (PbI 2 ): photography; cloud seeding to produce rain
lead naphthenate (Pb(C 7 H 12 O 2 )): wood preservative; insecticide; additive for lubricating oil; paint and varnish drier
lead phosphite (2PbO ○ PbHPO 3 ): used to screen out ultraviolet radiation in plastics and paints
lead stearate (Pb(C 18 H 35 O 2 ) 2 ): used to make soaps, greases, waxes, and paints; lubricant; drier for paints and varnishes
lead telluride (PbTe): used to make semiconductors, photoconductors, and other electronic equipment
Health effects
The health effects of lead have become much better understood since the middle of the 20th century. At one time, the metal was regarded as quite safe to use for most applications. Now lead is known to cause both immediate and long-term health problems, especially with children. It is toxic when swallowed, eaten, or inhaled.
Young children are most at risk from lead poisoning. Some children have a condition known as pica. They have an abnormal desire to eat materials like dirt, paper, and chalk. Children with pica sometimes eat paint chips off walls. At one time, many interior house paints were made with lead compounds. Thus, crawling babies or children with pica ran the risk of eating large amounts of lead and being poisoned.
Some symptoms of lead poisoning include nausea, vomiting, extreme tiredness, high blood pressure, and convulsions (spasms). Over a long period of time, these children often suffer brain damage. They lose the ability to carry out normal mental functions.
Other forms of lead poisoning can also occur. For example, people who work in factories where lead is used can inhale lead fumes. The amount of fumes inhaled at any one time may be small. But over months or years, the lead in a person's body can build up. This kind of lead poisoning can lead to nerve damage and problems with the gastrointestinal system (stomach and intestines).
Lead causes both immediate and longterm health problems, especially with children. It is toxic when swallowed, eaten, or inhaled.
Today, there is an effort to reduce the use of lead in consumer products. For instance, older homes are often tested for lead paint before they are resold. Lead paint has also been removed from older school buildings.