URANIUM

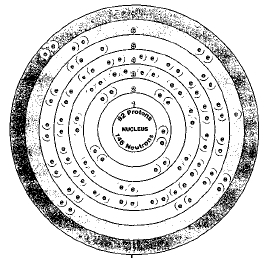
Overview
Uranium is the heaviest and last naturally occurring element in the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. Uranium occurs near the beginning of the actinide family. The actinide family consists of elements with atomic numbers 90 through 103.
At one time, uranium was considered to be a relatively unimportant element. It had a few applications in the making of stains and dyes, in producing specialized steels, and in lamps. But annual sales before World War II (1939-45) amounted to no more than a few hundred metric tons of the metal and its compounds.
SYMBOL
U
ATOMIC NUMBER
92
ATOMIC MASS
238.0289
FAMILY
Actinide
PRONUNCIATION
yUH-RAY-nee-um
Then, a dramatic revolution occurred. Scientists discovered that one form of uranium will undergo nuclear fission. Nuclear fission is the process in which the nuclei of large atoms break apart. Large amounts of energy and smaller atoms are produced during fission. The first application of this discovery was in the making of nuclear weapons, such as the atomic bomb. After the war, nuclear power plants were built to make productive use of nuclear fission. Nuclear power plants convert the energy released by fission to electricity. Today, uranium is regarded as one of the most important elements for the future of the human race.
Discovery and naming
Credit for the discovery of uranium is usually given to German chemist Martin Klaproth (1743-1817). During the late 1780s, Klaproth was studying a common and well-known ore called pitchblende. At the time, scientists thought that pitchblende was an ore of iron and zinc.
During his research, however, Klaproth found that a small portion of the ore did not behave the way iron or zinc would be expected to behave. He concluded that he had found a new element and suggested the name uranium for the element. The name was given in honor of the Uranus, a planet that had been discovered only a few years earlier, in 1781.
For some time, scientists believed that Klaproth had isolated uranium. Eventually they realized he had found uranium oxide (UO 2 ), a compound of uranium. It was not until a half century later, in fact, that the pure element was prepared. In 1841, French chemist Eugène-Melchior Peligot (1811-90) produced pure uranium from uranium oxide.
Early researchers did not know that uranium was radioactive. In fact, radioactivity was not discovered until 1898. Radioactivity is the tendency of an isotope or element to break down and give off radiation.
Physical properties
Uranium is a silvery, shiny metal that is both ductile and malleable. Ductile means capable of being drawn into thin wires. Malleable means capable of being hammered into thin sheets. Its melting point is 1,132.3°C (2,070.1°F) and its boiling point is about 3,818°C (6,904°F). Its density is about 19.05 grams per cubic centimeter.
Chemical properties
Uranium is a relatively reactive element. It combines with nonmetals such as oxygen, sulfur, chlorine, fluorine, phosphorus, and bromine. It also dissolves in acids and reacts with water. It forms many compounds that tend to have yellowish or greenish colors.
Occurrence in nature
Uranium is a moderately rare element. Its abundance is estimated to be about 1 to 2 parts per million, making it about as abundant as bromine or tin. The most common ore of uranium is pitchblende, although it also occurs in other minerals, such as uraninite, carnotite, uranophane, and coffinite.
Isotopes
All isotopes of uranium are radioactive. Three of these occur naturally, uranium-234, uranium-235, uranium-238. By far the most common is uranium-238, making up about 99.276% of uranium found in the Earth's crust. Uranium-238 also has the longest half life, about 4,468,000,000 years.
Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons
The half life of a radioactive element is the time it takes for half of a sample of the element to break down. Imagine that the Earth's crust contains 100 million tonnes of uranium-238 today. Only half of a uranium-238 sample would remain 4,468,000,000 years from now (one half life). The remainder would have changed into other isotopes.
About a dozen other isotopes of uranium have been made artificially.
Extraction
Uranium is mined in much the same way iron is. Ore is removed from the earth, then treated with nitric acid to make uranyl nitrate (UO 2 (NO 3 ) 2 ). This compound is converted to uranium
Uses and compounds
Uranium compounds have been used to color glass and ceramics for centuries. Scientists have found that glass made in Italy as early as A.D. 79 was colored with uranium oxide. They have been able to prove that the coloring was done intentionally.
Some uranium compounds were used for this purpose until quite recently. In fact, a popular type of dishware known as "Fiesta Ware" made in the 1930s and 1940s sometimes used uranium oxide as a coloring material. Other glassware, ceramics, and glazes also contained uranium oxide as a coloring agent.
Uranium compounds have had other limited uses. For example, they have been used as mordants in dyeing operations. A mordant
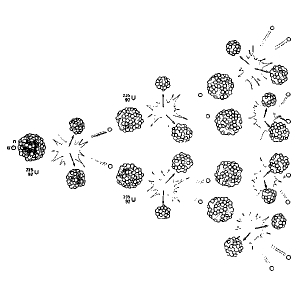
None of these applications is of very much importance today, however. By far the most important application is in nuclear weapons and nuclear power plants. The reason for this importance is that one isotope of uranium, uranium-235, undergoes nuclear fission.
Nuclear fission is the process by which neutrons are fired at a target. The target is usually made of uranium atoms. When neutrons hit the target, they cause the nuclei of uranium atoms to break apart. Smaller elements are formed and very Large amounts of energy are given off.
When this reaction is carried out with no attempt to capture or control the energy, an enormous explosion takes place. This release of nuclear energy accounts for the power of a nuclear weapon such as an atomic bomb. In reactors, the energy released during fission is used to boil water. Steam is produced and is converted to electricity. The controlled release of nuclear energy takes place in a nuclear power plant.
Separating twins to make energy
S uppose neutrons are fired at a big block of uranium metal. Would nuclear fission occur? Would this be the way to make an atomic bomb? Could this process be used in a nuclear power plant?
The answer to all these questions is no. Only one isotope of uranium undergoes nuclear fission, uranium-235. The most common isotope, uranium-238, does not undergo fission. There is no way to make a bomb or a nuclear power plant with a chunk of natural uranium metal.
It is necessary is to increase the percentage of uranium-235 in the metal. As a chunk of uranium metal contains more uranium-235, it is more likely to undergo nuclear fission.
In making a bomb or a power plant, then, the first step is to separate the isotopes of uranium from each other. The goal is to produce more uranium-235 and less uranium-238.
hat goal sounds easy, but it is very difficult to do. All isotopes of uranium behave very much alike. They have the same chemical properties. The only way they differ from each other is by weight. An atom of uranium-238, for example, weighs about 1 percent more than an atom of uranium-235. That's not much of a difference.
Scientists separate these isotopes in a centrifuge. A centrifuge is a machine that spins containers of materials at very high speeds. They are like some of the rides at an amusement park. A person sits in a compartment at the end of a long arm. When the ride is turned on, the compartment spins around faster and faster.
In a centrifuge, heavier objects spin farther out than do lighter objects. A mixture of uranium-235 and uranium-238 can be separated slightly in a centrifuge. But the separation is not very good because the isotopes weigh almost the same amount.
In practice, a mixture of isotopes must be centrifuged many times. Each time, the separation gets better.
Scientists prepare enriched uranium by this method. Enriched uranium contains more uranium-235 and less uranium-238. Enriched uranium was used to make atomic bombs and is now used in nuclear power plants. It contains enough uranium-235 to allow nuclear fission to occur.
Today, over 400 nuclear power plants exist worldwide, producing about 17 percent of all electricity. Many people believe nuclear power will be more important in the future as the world's supply of coal, oil, and natural gas eventually runs out.
Other people are concerned about the dangers of nuclear power. The radiation released and radioactive wastes produced by nuclear power plants have made them unpopular in the United States. No new nuclear generators have been built for over ten years. It is not clear what the future of nuclear power plants in the United States will be.
Uranium poses an exceptional risk in powdered form. In this form, it tends to catch fire spontaneously.
Health effects
Since it is a radioactive element, uranium must be handled with great care. In addition, it poses an exceptional risk in powdered form. In this form, it tends to catch fire spontaneously.
** ...button of uranium-235 held..." = uranium235 is held in (apparently) human hands !? :-o