CARBON

Overview
Carbon is an extraordinary element. It occurs in more different forms than any other element in the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. More than ten million compounds of carbon are known. No other element, except for hydrogen, occurs in even a fraction of that number of compounds.
As an element, carbon occurs in a striking variety of forms. Coal, soot, and diamonds are all nearly pure forms of carbon. Carbon also occurs in a form, discovered only recently, known as fullerenes or buckyballs. Buckyball carbon holds the promise for opening a whole new field of chemistry (see accompanying sidebar).
Carbon occurs extensively in all living organisms as proteins, fats, carbohydrates (sugars and starches), and nucleic acids.
SYMBOL
C
ATOMIC NUMBER
6
ATOMIC MASS
12.01115
FAMILY
Group 14 (IVA)
Carbon
PRONUNCIATION
CAR-bun
Carbon is such an important element that an entirely separate field of chemistry is devoted to this element and its compounds. Organic chemistry is the study of carbon compounds.
Discovery and naming
Humans have been aware of carbon since the earliest of times. When cave people made a fire, they saw smoke form. The black color of smoke is caused by unburned specks of carbon. The smoke may have collected on the ceiling of their caves as soot.
Later, when lamps were invented, people used oil as a fuel. When oil burns, carbon is released in the reaction, forming a sooty covering on the inside of the lamp. That form of carbon became known as lampblack. Lampblack was also often mixed with olive oil or balsam gum to make ink. And ancient Egyptians sometimes used lampblack as eyeliner.
One of the most common forms of carbon is charcoal. Charcoal is made by heating wood in the absence of air so it does not catch fire. Instead, it gives off water vapor, leaving pure carbon. This method for producing charcoal was known as early as the Roman civilization (509 B.C.-A.D. 476).
French physicist René Antoine Ferchault Reaumur (1683-1757) believed carbon might be an element. He studied the differences between wrought iron, cast iron, and steel. The main difference among these materials, he said, was the presence of a "black combustible material" that he knew was present in charcoal.
Carbon was officially classified as an element near the end of the eighteenth century. In 1787, four French chemists wrote a book outlining a method for naming chemical substances. The name they used, carbone, is based on the earlier Latin term for charcoal, charbon.
Coal, soot (nearly pure carbon), and diamonds are all nearly pure forms of carbon.
Physical properties
Carbon exists in a number of allotropic forms. Allotropes are forms of an element with different physical and chemical properties. Two allotropes of carbon have crystalline structures: diamond and graphite. In a crystalline material, atoms are arranged in a neat orderly pattern. Graphite is found in pencil "lead" and ball-bearing lubricants. Among the non-crystalline allotropes of carbon are coal, lampblack, charcoal, carbon black, and coke. Carbon black is similar to soot. Coke is nearly pure carbon formed when coal is heated in the absence of air. Carbon allotropes that lack crystalline structure are amorphous, or without crystalline shape.
The allotropes of carbon have very different chemical and physical properties. For example, diamond is the hardest natural substance known. It has a rating of 10 on the Mohs scale. The Mohs scale is a way of expressing the hardness of a material. It runs from 0 (for talc) to 10 (for diamond). The melting point of diamond is about 3,700°C (6,700°F) and its boiling point is about 4,200°C (7,600°F). Its density is 3.50 grams per cubic centimeter.
On the other hand, graphite is a very soft material. It is often used as the "lead" in lead pencils. It has a hardness of 2.0 to 2.5 on the Mohs scale. Graphite does not melt when heated, but sublimes at about 3,650°C (6.600°F). Sublimination is the process by which a solid changes directly to a gas when heated, without first changing to a liquid. Its density is about 1.5 to 1.8 grams per cubic centimeter. The numerical value for these properties varies depending on where the graphite originates.
The amorphous forms of carbon, like other non-crystalline materials, do not have clear-cut melting and boiling points. Their densities vary depending on where they originate.
Chemical properties
Carbon does not dissolve in or react with water, acids, or most other materials. It does, however, react with oxygen. It burns in air to produce carbon dioxide (CO 2 ) and carbon monoxide (CO). The combustion (burning) of coal gave rise to the Industrial Revolution (1700-1900).
Another highly important and very unusual property of carbon is its ability to form long chains. It is not unusual for two atoms of an element to combine with each other. Oxygen (O 2 ), nitrogen (N 2 ), hydrogen (H 2 ), chlorine (Cl 2 ), and bromine (Br 2 ) are a few of the elements that can do this. Some elements can make even longer strings of atoms. Rings of six and eight sulfur atoms (S 6 and S 8 ), for example, are not unusual.
Carbon has the ability to make virtually endless strings of atoms. If one could look at a molecule of almost any plastic, for example, a long chain of carbon atoms attached to each other (and to other atoms as well) would be evident. Carbon chains can be even more complicated. Some chains have side chains hanging off them.

There is almost no limit to the size and shape of molecules that can be made with carbon atoms. (See accompanying diagrams.)
Buckyballs are a recently discovered form of pure carbon. These spheres are made up of exactly 60 linked carbon atoms.
Occurrence in nature
Carbon is the sixth most common element in the universe and the fourth most common element in the solar system. It is the second most common element in the human body after oxygen. About 18 percent of a person's body weight is due to carbon.
The black color of smoke is caused by unburned specks of carbon.
Carbon is the 17th most common element in the Earth's crust. Its abundance has been estimated to be between 180 and 270 parts per million. It rarely occurs as a diamond or graphite.

Both allotropes are formed in the earth over millions of years, when dead plant materials are squeezed together at very high temperatures. Diamonds are usually found hundreds or thousands of feet beneath the earth's surface. Africa has many diamond mines.
Carbon also occurs in a number of minerals. Among the most common of these minerals are the carbonates of calcium (CaCO 3 ) and magnesium (MgCO 3 ). Carbon also occurs in the form of carbon dioxide (CO 2 ) in the atmosphere. Carbon dioxide makes up only a small part of the atmosphere (about 300 parts per million), but it is a crucial gas. Plants use carbon dioxide in the atmosphere in the process of photosynthesis. Photosynthesis is the process by which plants convert carbon dioxide and water to carbohydrates (starches and sugars). This process is the source of life on Earth.
Carbon also occurs in coal, oil, and natural gas. These materials are often known as fossil fuels. They get that name because of the way they were formed. They are the remains of plants and animals that lived millions of years ago. When they died, they fell into water or were trapped in mud. Over millions of years, they slowly decayed. The products of that decay process were coal, oil, and natural gas.
Some forms of coal are nearly pure carbon. Oil and natural gas are made primarily of hydrocarbons, which are compounds made of carbon and hydrogen.
Isotopes
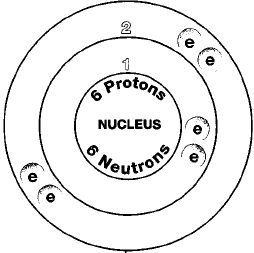
Three isotopes of carbon occur in nature, carbon-12, carbon-13, and carbon-14. One of these isotopes, carbon-14, is radioactive. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Five artificial radioactive isotopes of carbon are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Artificial radioactive isotopes can be made by firing very small particles (such as protons) at atoms. These particles stick in the atoms and make them radioactive.
Carbon-14 has some limited applications in industry. For example, it can be used to measure the thickness of objects, such as sheets of steel. The steel must always be the same thickness.
Carbon is the sixth most common element in the universe and the fourth most common element in the solar system.
In this process, a small sample of carbon-14 is placed above the conveyor belt carrying the steel sheet. A detection device is placed below the sheet. The detection device counts the amount of radiation passing through the sheet. If the sheet gets thicker, Less radiation gets through. If the sheet gets thinner, more radiation gets through. The detector records how much radiation passes through the sheet. If the amount becomes too high or too low, the conveyor belt is turned off. The machine making the sheet is adjusted to produce steel of the correct thickness.
The most important use of carbon-14 is in finding the age of old objects (see accompanying sidebar for more information).
Extraction
Diamond, graphite, and other forms of carbon are taken directly from mines in the earth. Diamond and graphite can also be made in laboratories. Synthetic diamonds, for example, are made by placing pure carbon under very high pressures (about 800,000 pounds per square inch) and temperatures (about 2,700°C). The carbon is heated and squeezed in the same way organic material is heated and squeezed in the earth. Today, about a third of all diamonds used are synthetically produced.
Carbon-14 dating
W hen an organism is alive, it takes in carbon dioxide from the air around it. Most of that carbon dioxide is made of carbon-12, but a tiny portion consists of carbon-14. So the living organism always contains a very small amount of radioactive carbon, carbon-14. A detector next to the living organism would record radiation given off by the carbon-14 in the organism.
When the organism dies, it no longer takes in carbon dioxide. No new carbon-14 is added, and the old carbon-14 slowly decays into nitrogen. The amount of carbon-14 slowly decreases as time goes on. Over time, less and less radiation from carbon-14 is produced. The amount of carbon-14 radiation detected for an organism is a measure, therefore, of how long the organism has been dead. This method of determining the age of an organism is called carbon-14 dating.
The decay of carbon-14 allows archaeologists (people who study old civilizations) to find the age of once-living materials. Measuring the amount of radiation remaining indicates the approximate age.
Uses
There are many uses for carbon's two key allotropes, diamond and graphite. Diamonds are one of the most beautiful and expensive gemstones in the world. But they also have many industrial uses. Because they are so hard they are used to polish, grind, and cut glass, metals, and other materials. The bit on an oil-drilling machine may be made of diamonds. The tool used to make thin tungsten wires is also made of diamonds.
Synthetic diamonds are more commonly used in industry than in jewelry. Industrial diamonds do not have to be free of flaws, as do jewelry diamonds.
Graphite works well as pencil lead because it rubs off easily. It is also used as a lubricant. Graphite is added to the space between machine parts that rub against each other. The graphite allows the parts to slide over each other smoothly.
Graphite is also used as a refractory. Refractory material can withstand very high temperatures by reflecting heat away from itself. Refractory materials are used to line ovens used to maintain high temperatures.
Graphite is used in nuclear power plants. A nuclear power plant converts nuclear energy to electrical power. Graphite acts as a moderator by slowing down the neutrons used in the nuclear reaction.
Graphite is used to make black paint, in explosives and matches, and in certain kinds of cathode ray tubes, like the ones used in television sets.
Amorphous forms of carbon have many uses. These include the black color in inks, pigments (paints), rubber tires, stove polish, typewriter ribbons, and phonograph records.
One form of carbon is known as activated charcoal. The term activated means that the charcoal has been ground into a very fine powder. In this form, charcoal can remove impurities from liquids that pass through. For example, activated charcoal removes color and odor from oils and water solutions.
The decay of carbon-14 allows archaeologists (people who study old civilizations) to find the age of once-living materials.
Compounds
Carbon dioxide (CO 2 ) is used to make carbonated beverages (it's the fizz in soda pop and beer), in fire extinguishers, and as a propellant in aerosol products. A propellant is a gas that pushes liquids out of a spray can, such as those used for deodorant or hair spray. Carbon dioxide can also be frozen to a solid called dry ice. It is widely used as a way of keeping objects cold.

Carbon monoxide (CO) is another compound formed between carbon and oxygen. Carbon monoxide is a very toxic gas produced when something burns in a limited amount of air. Carbon monoxide is always formed when gasoline burns in the engine of an automobile and is a common part of air pollution. Old heating units can produce carbon monoxide. This colorless and odorless gas can cause headaches, illness, coma, or even death.
Carbon monoxide has a few important industrial uses. It is often used to
obtain a pure metal from the ore of that metal:
It would take a very large book to describe all the uses of organic compounds, which are divided into a number of families. An organic family is a group of organic compounds with similar structures and properties. The largest organic family is the hydrocarbons, compounds that contain only carbon and hydrogen. Methane, or natural gas (CH 4 ), ethane (C 2 H 6 ), propane (C 3 H 8 ), ethylene (C 2 H 4 ), and benzene (C 6 H 6 ) are all hydrocarbons.
Hydrocarbons are used as fuels. Gas stoves burn natural gas, which is mostly methane. Propane gas is a popular camping fuel, used in small stoves and lanterns. Another important use of hydrocarbons is in the production of more complicated organic compounds.
Buckyballs and nanotubes

In the 1980s, chemists discovered a new allotrope of carbon. The carbon atoms in this allotrope are arranged in a sphere-like form of 60 atoms. The form resembles a building invented by American architect Buckminster Fuller (1895-1983). The building is known as a geodesic dome.
Each of the points on the dome is occupied by one carbon atom. The discoverers named the new form of carbon buckminsterfullerene in honor of Fuller. That name is too long to use in everyday conversation so it is usually shortened to fullerene or buckyball.
The discovery of the fullerene molecule was very exciting to chemists. They had never seen a molecule like it. They have been studying ways of working with this molecule. One interesting technique has been to cut open just one small part of the molecule. Then they cut open a small part on a second molecule. Finally, they join the two buckyballs together. They get a double-buckyball.
Repeating this process over and over could result in triple-buckyballs, quadruple-buckyballs, and so on. As this process is repeated, the buckyball becomes a long narrow tube called a nanotube. Nanotubes are long, thin, and extremely tiny tubes somewhat like a drinking straw or a long piece of spaghetti.
Scientists are beginning to find ways of using nanotubes. One idea is to run a thin chain of metal atoms through the center of a nanotube. This allows it to act like a tiny electrical wire. Nanotubes may completely change many devices that will be made in the future.
Other organic families contain carbon, hydrogen, and oxygen. Methyl alcohol (wood alcohol) and ethyl alcohol (grain alcohol) are the most common members of the alcohol family.
Methyl alcohol is used to make other organic compounds and as a solvent (a substance that dissolves other substances). Ethyl alcohol is used for many of the same purposes. It is also the alcohol found in beer, wine, and hard liquor, such as whiskey and vodka.
All alcohols are poisonous but some alcohols are more poisonous than others. If not drunk in moderation, alcoholic beverages can damage the body and brain. And, if drunk in large quantities, they can cause death. Methyl alcohol is more toxic than ethyl alcohol. People who have drunk methyl alcohol by mistake have died.
The list of everyday products made from organic compounds is very long. It includes drugs, artificial fibers, dyes, artificial colors and flavors, food additives, cosmetics, plastics of all kinds, detergents, synthetic rubber, adhesives, antifreeze, pesticides and herbicides, synthetic fuels, and refrigerants.
Health effects
Carbon is essential to life. Nearly every molecule in a living organism contains carbon. The study of carbon compounds that occur in living organisms is called biochemistry (bio- = life + -chemistry ).
Carbon can also have harmful effects on organisms. For example, coal miners sometimes develop a disease known as black lung. The name comes from the appearance of the miner's lungs. Instead of being pink and healthy, the miner's lungs are black. The black color is caused by coal dust inhaled by the miner. The longer a miner works digging coal, the more the coal dust is inhaled. That worker's lungs become more and more black.
Color is not the problem with black lung disease however. The coal dust in the lungs blocks the tiny holes through which oxygen gets into the lungs. As more coal dust accumulates, more holes are plugged up, making it harder for the miner to breathe. Many miners eventually die from black lung disease because they lose the ability to breathe.
Carbon is essential to life. Nearly every molecule in a living organism contains carbon.
Carbon monoxide poisoning is another serious health problem. Carbon monoxide is formed whenever coal, oil, or natural gas bums. For example, the burning of gasoline in cars and trucks produces carbon monoxide. Today, almost every person in the United States inhales some carbon monoxide every day.
Small amounts of carbon monoxide are not very dangerous. But larger amounts cause a variety of health problems. At low levels, carbon monoxide causes headaches, dizziness, nausea, and loss of balance. At higher levels, a person can lose consciousness. At even higher levels, carbon monoxide can cause death.
Nick