STRONTIUM

Overview
Strontium is a member of the alkaline earth metals. The alkaline earth metals make up Group 2 (IIA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. Other alkaline metals include beryllium, magnesium, calcium, barium, and radium. Strontium occupies a middle position in the family. Chemically, it is more active than calcium or magnesium, above it in the periodic table. But it is less active than barium, below it in Group 2.
The existence of strontium was first recognized in 1790 by Irish physician Adair Crawford (1748-95). However, the element was not prepared in pure form until nearly 20 years later by English chemist Humphry Davy (1778-1829). (See sidebar on Davy in the calcium entry in Volume 1.)
By far the major use of strontium is in the production of color television tubes. It is also used in the manufacture of ceramics and specialty glass. One of its radioactive isotopes is used in industry and medical studies.
SYMBOL
Sr
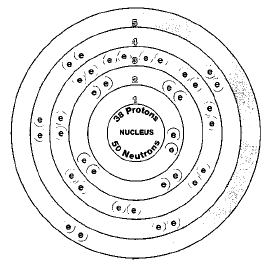
ATOMIC NUMBERM
38
ATOMIC MASS
87.62
FAMILY
Group 2 (IIA)
Alkaline earth metal
PRONUNCIATION
STRONT-she-um
Discovery and naming
Adair Crawford was trained as a physician. However, he was also interested in chemical research. For a period of time, he was on the staff at St. Thomas's Hospital in London, England, and a professor of chemistry at Woolwich University.
In 1790, he began studing certain minerals that were on display at St. Thomas's. These minerals were thought to be a form of baryte. Baryte is a mineral from which the element barium is obtained.
But Crawford found that some of the minerals did not behave as he expected. They did not have the properties of barium minerals. He concluded that the minerals contained a new element. He called the element strontia. He named it after a lead mine in Strontia, Scotland, from which the samples came.
Strontia was later found to be a compound of strontium and
oxygen.
In 1808, Davy found a way to produce pure strontium metal. He passed an
electric current through molten (melted) strontium chloride. The electric
current broke the compound into its two elements:
Physical properties
Strontium is a silvery-white, shiny metal. When exposed to air, it combines with oxygen to form a thin film of strontium oxide (SrO). The film gives the metal a yellowish color.
Strontium has a melting point of about 757°C (1,395°F) and a boiling point of 1,366°C (2,491°F). Its density is 2.6 grams per cubic centimeter.
Chemical properties
Strontium is so active it must be stored under kerosene or mineral oil. In
this way, the metal does not come into contact with air. In a finely
divided or powdered form, strontium catches fire spontaneously and bums
vigorously. Strontium is active enough to combine even with
hydrogen
and
nitrogen
when heated. The compounds formed are strontium hydride (SrH
2
) and strontium nitride (Sr
3
N
2
). Strontium also reacts with cold water and with acids to release
hydrogen gas:
Occurrence in nature
Strontium is a relatively abundant element in the Earth's crust. It ranks about 15th among the elements found in the Earth. That makes it about as abundant as fluorine and its alkaline earth partner, barium.
The most common minerals containing strontium are celestine and strontianite. Celestine contains primarily strontium sulfate (SrSO 4 ), while strontianite contains mostly strontium carbonate (SrCO 3 ). Important world sources of strontium are Mexico, Spain, Turkey, and Iran. A small amount of strontium is also obtained from mines in California and Texas.
Isotopes
Four isotopes of strontium occur in nature. They are strontium-84, strontium-86, strontium-87, and strontium-88. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
About ten radioactive isotopes of strontium are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
One radioactive isotope of strontium, strontium-90, is of special interest. It is a toxic substance which, at one time, was the cause of great concern because of its connection to atomic bomb testing. (See accompanying sidebar.)
Today, strontium-90 has a number of useful applications. For example, it is used to monitor the thickness of materials. Metal sheeting for construction must be the same thickness throughout. The sheeting is carried along on a conveyor belt beneath a small container of strontium-90. The isotope gives off radiation, some of which passes through the metal sheeting. The thicker the sheeting, the less radiation gets through. The thinner the sheeting, the more radiation gets through. A radiation detector is placed below the conveyor belt. The detector measures the amount of radiation passing through the sheeting. An inspector monitors the reading and makes adjustments to the manufacturing equipment to maintain the right thickness.
Strontium-90 is used for a number of other industrial applications, all based on the same principle. For instance, strontium-90 is used to measure the density of silk and tobacco products.
Strontium-90 has medical applications. A recent advance is to use the isotope for the control of pain. People who have cancer of the bone often experience terrible pain. At one time, the only treatment was medication. But those drugs often had unpleasant side-effects, such as nausea, dizziness, or depression.
Poison from the sky: strontium-90
S trontium-90 is a radioactive isotope produced during the explosion of atomic weapons, such as an atomic bomb. In the 1950s and 1960s, the United States, the then-Soviet Union, China, and a few other nations tested atomic bombs in the atmosphere. Whenever one of these bombs exploded, some strontium-90 was thrown high into the atmosphere. After a short time, the strontium-90 settled to the ground where it was absorbed by growing plants. When cattle, sheep, and other domestic animals ate the plants, they also took strontium-90 into their bodies.
Strontium is just below calcium on the periodic table. That means that strontium behaves in much the same way that calcium does. Calcium eaten by humans and animals goes primarily to building bones and teeth. TV advertisements frequently recommend that young children drink milk. That's because milk contains calcium. It is used to build bones and teeth in growing children.
So any strontium that enters an animal's body is also used to build bones and teeth. The bad news is that strontium-90 is radioactive. It gives off radiation that kills or damages living cells. It can also cause those cells to begin growing out of control. Out-of-control cells lead to cancer. Strontium-90 in bones and teeth is a built-in time bomb. As long as it remains in the body, it has the potential for causing cancer in people and animals.
The threat posed by strontium-90 is one reason that nations agreed to begin testing nuclear weapons underground. It also helped world leaders realize that they needed to stop the testing of nuclear weapons entirely. It led to some degree to the agreements signed in the 1980s among the United States, Soviet Union, and other nations to give up atomic bomb testing entirely.
Injecting strontium-90 into a person's body is now an alternative to the use of drugs. The strontium-90 deposits in the

There are other medical applications for radioactive strontium isotopes. Strontium-90 is used to treat a variety of eye disorders. And strontium-85 and strontium-87m are used to study the condition of bones in a person's body.
Extraction
Most strontium metal is still obtained by the method used by Davy. An electric current is passed through molten (melted) strontium chloride.
Uses and compounds
Strontium and its compounds have relatively few commercial uses. The pure metal is sometimes combined with other metals to form alloys. An alloy is made by melting and mixing two or more metals. The mixture has different properties than the individual metals. Compounds of strontium are sometimes used to color glass and ceramics. They give a beautiful red color to these materials. Strontium compounds also provide the brilliant red color of certain kinds of fireworks.
Health effects
Most strontium compounds are regarded as harmless to plants and animals. A few, such as strontium chloride (SrCl 2 ) and strontium iodide (SrI 2 ), are somewhat toxic.
Comment about this article, ask questions, or add new information about this topic: