NITROGEN
Overview
Nitrogen is the first member in Group 15 (VA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. Nitrogen is in a family group named after itself. Other elements in the nitrogen family are phosphorus, arsenic, antimony, and bismuth.
Nitrogen is one of the most interesting of all chemical elements. It is not a very active element. It combines with relatively few other elements at room temperature. Yet, the compounds of nitrogen are enormously important both in living organisms and in industrial applications. Five of the top fifteen chemicals that are produced synthetically by chemical producers are compounds of nitrogen or the element itself. How does such an inactive element end up with so many important compounds?
Nitrogen makes up more than three-quarters of the Earth's atmosphere. It is also found in a number of rocks and minerals in the Earth's surface. It ranks about number 32 among the elements in terms of abundance in the Earth's crust.
SYMBOL
N
ATOMIC NUMBER
7
ATOMIC MASS
14.0067
FAMILY
Group 15 (VA)
Nitrogen
PRONUNCIATION
NYE-tru-jun
Nitrogen was discovered by a number of chemists at about the same time, approximately 1772. But it was not until the early part of the twentieth century, when chemists learned how to make compounds of nitrogen, that the most important uses of the element became known.
By far the most notable use of nitrogen is in the production of ammonia (NH 3 ). Ammonia is used to make other compounds, such as ammonium sulfate ((NH 4 ) 2 SO 4 ), ammonium nitrate (NH 4 NO 3 ), urea (CO(NH 2 ) 2 ), and nitric acid (HNO 3 ). These compounds are primarily used to make synthetic fertilizer. Both elemental nitrogen and nitrogen compounds have a number of important industrial uses.
Discovery and naming
Gases were poorly understood by chemists until the late 1700s. What is air "made of?" That question is difficult to answer for a number of reasons. First, air cannot really be "seen." In fact, it took chemists many years to figure out how to capture air so that they could study it. Also, is ordinary "air" an element or a compound? For many centuries, philosophers said that air was an element. They could not imagine how anything as basic as air could be made of other materials.
Also, is ordinary air different from other kinds of "airs" seen in nature? For example, "air" sometimes comes bubbling out of the ground near oil wells. Today, scientists know that kind of "air" as methane gas (CH 4 ). But early chemists were not sure how "oil air" differed from ordinary air.
Some important breakthroughs in the study of air occurred in the 1770s. The key was a simple experiment that science students still do today. The experiment begins with an empty bottle being turned upside down in a pan of water. The air in the bottle cannot get out.
If a burning candle is placed inside the bottle with the trapped air, the water rises just a bit. Why does this happen? Early chemists thought that a part of the air was used up as the candle burns. Today, they know that part of the air is oxygen gas. Another part of the air is left behind. That part does not disappear when the candle burns.
This simple experiment shows that air is composed of (at least) two different elements: oxygen and something else. One of the first people to discover what the "something else" is was Scottish physician and chemist Daniel Rutherford (1749-1819). Rutherford carried out an experiment like the candle-in-a-bottle research just described.
Some of the greatest chemists of the time were working on this problem at the same time that Rutherford made his discovery. English chemist Henry Cavendish (1731-1810) probably discovered nitrogen before Rutherford did, but did not publish his findings. And in science, the first person to publish the results of an experiment usually gets credit for the work.
It seems likely that English chemist Joseph Priestley (1733-1804) and Swedish chemist Carl Wilhelm Scheele (1742-86) also discovered nitrogen in the early 1770s. (See sidebar on Scheele in the chlorine entry in Volume 1.)
Chemists debated about the name of this new element for some time. Antoine-Laurent Lavoisier (1743-94), a French chemist and the "father of modern chemistry," preferred the name azote meaning "without life." Lavoisier chose this name because nitrogen does not support breathing, the way oxygen does. (See sidebar on Lavoisier in the oxygen entry in Volume 2.)
The modern name of nitrogen was first suggested in 1790 by French chemist Jean Antoine Claude Chaptal (1756-1832). This name made sense to chemists when they realized that the new gas was present in both nitric acid and nitrates. Thus, nitrogen means "nitrate and nitric acid" (nitro-) and "origin of" (-gen).
Physical properties
Nitrogen is a colorless, odorless, tasteless gas with a density of 1.25046 grams per liter. By comparison, the density of air is about 1.29 grams per liter. Nitrogen changes from a gas into a liquid at a temperature of -195.79°C (-320.42°F) . It changes from a liquid to a solid at a temperature of -210.01°C (-346.02°F). When it freezes, it becomes a white solid that looks like snow. Nitrogen is slightly soluble in water. About two liters of nitrogen can be dissolved in 100 liters of water.
Chemical properties
At room temperature, nitrogen is a very inactive gas. It does not combine
with oxygen,
hydrogen,
or most other elements. Nitrogen will combine with oxygen, however, in
the presence
of lightning or a spark. The electrical energy from either of those
sources causes nitrogen and oxygen to form nitric oxide:
Nitric oxide is more active than free nitrogen. For example, nitric oxide combines with oxygen and water in the atmosphere to make nitric acid. When it rains, nitric acid is carried to the earth. There it combines with metals in the Earth's crust. Compounds known as nitrates and nitrites are formed.
Changing nitrogen as an element to nitrogen in compounds is called nitrogen fixation. The reaction between nitrogen and oxygen in the air when lightning strikes is an example of nitrogen fixation.
Certain bacteria have developed methods for fixing nitrogen. These bacteria live on the root hairs of plants. They take nitrogen out of air dissolved in the ground and convert it to compounds, such as nitrates. Those nitrates are used to make protein molecules, compounds vital to the building and growth of cells.
Plants, animals, and humans do not have the ability to fix nitrogen. All living organisms on Earth depend on soil bacteria to carry out this process. Plants can grow because the bacteria fix nitrogen for them. They use the fixed nitrogen to make proteins. Animals and humans can survive because they eat plants. They also depend on the soil bacteria that allow plants to make proteins. So all living creatures rely on soil bacteria to fix their nitrogen for them and, therefore, to survive.
Occurrence in nature
Nitrogen is a fairly common element in the Earth's crust. It occurs primarily as nitrates and nitrites. Nitrogen is by far the most important element in the Earth's atmosphere. It makes up 78.084 percent of the atmosphere.
Nitrogen combines with oxygen in the presence of lightning or a spark. The electrical energy from those sources causes nitrogen and oxygen to form nitric oxide.
Isotopes
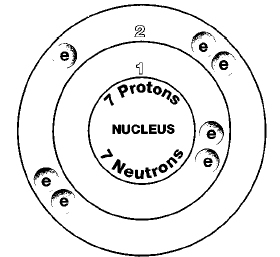
Two naturally occurring isotopes of nitrogen exist, nitrogen-14 and nitrogen-15. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Five radioactive isotopes of nitrogen are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
None of the radioactive isotopes of nitrogen has any important commercial use. However, nitrogen-15 is used quite often in tracer studies. A tracer is a radioactive isotope whose presence in a system can be detected. Normally, tracer studies use radioactive isotopes. These isotopes give off radiation that can be detected with instruments. Nitrogen-15 is used for a different reason. A compound made with nitrogen-15 will weigh just a little bit more than one made with nitrogen-14. There are simple chemical methods for detecting whether a heavier compound or a lighter one is present in a system. Thus, nitrogen-15 can be used to trace the path of nitrogen through a system.
Extraction
Nitrogen is almost always made from liquid air. Liquid air is made by cooling normal atmospheric air to very low temperatures. As the temperature drops, the gases contained in air turn into liquids. At -182.96°C (-297.33°F) , oxygen changes from a gas into a Liquid. At -195.79°C (-320.42°F), nitrogen changes from a gas into a liquid. And so on. Eventually, all the gases in air can be made to liquefy (change into a liquid).
The reverse process also takes place. Suppose liquid air in a container warms up slowly. When its temperature reaches -195.79°C, liquid nitrogen changes back to a gas. A container can be put into place to catch the nitrogen as it boils off the liquid air. When the temperature reaches -182.96°C, oxygen changes from a liquid back to a gas. Another container can be put into place. The escaping oxygen can be collected. All of the gases in atmospheric air can be produced by this method.
Large amounts of nitrogen gas are produced in this way. In fact, nitrogen is second only to sulfuric acid in terms of production. In 1996, more than a trillion cubic feet of nitrogen gas were produced in the United States alone.
Uses
Nitrogen gas is used where an inert atmosphere is needed. An inert atmosphere is one that does not contain active elements. Ordinary air is not an inert atmosphere. It contains oxygen. Oxygen tends to react with other elements.
Suppose an ordinary light bulb were filled with air. When the bulb is turned on, an electric current runs through the metal filament (wire) inside the bulb. The filament gets very hot, begins to glow, and gives off light.
But a hot metal wire will react quickly with oxygen in ordinary air. The metal combines with oxygen to form a compound of the metal. The metal compound will not conduct an electric current. The bulb will "burn out" very quickly.
An easy solution to that problem is to use nitrogen instead of ordinary air in the light bulb. Nitrogen does not react with other elements very well, even when they get hot. The filament can get very hot, but the metal of which it is made will not combine with nitrogen gas. The nitrogen gas is an inert atmosphere for the bulb.
Another use for inert atmospheres is in protecting historic documents. Suppose the Declaration of Independence were simply left on top of a table for people to see. The paper and ink in the document would soon begin to react with oxygen in the air. They would both begin to decay. Before long, the document would begin to fall apart.
Instead, important documents like the Declaration of Independence are kept in air-tight containers filled with nitrogen gas. The documents are protected from oxygen and other gases in the air with which they might react.
Fairly simple methods are now available for changing nitrogen gas into liquid nitrogen. Liquid nitrogen is used to freeze other materials. The temperature of the nitrogen has to be reduced to -195.79°C (-320.42°F) for this change to occur.
Historic documents like the Declaration of Independence are protected in air-tight containers filled with nitrogen gas. This keeps them from oxygen and other gases that would cause them to decay.
Today, it is possible to buy large containers of liquid nitrogen. The liquid nitrogen can be used, then, to freeze other materials. For example, foods can be frozen simply by dipping them into large vats of liquid nitrogen. The frozen foods in a grocery store are usually produced this way. Liquid nitrogen can also
Compounds
Nitrogen is the starting point for an important group of compounds. First, nitrogen is combined with hydrogen to make ammonia (NH 3 ). The production of ammonia is sometimes called industrial nitrogen fixation.
The formation of ammonia from nitrogen and hydrogen is very difficult to accomplish. The two elements do not easily combine. Finding a way to make nitrogen and hydrogen combine was one of the great scientific discoveries of the twentieth century.
The nitrogen compound ammonia is used by most farmers in synthetic fertilizers to ensure large crops.
That discovery was made by German chemist Fritz Haber (1868-1934) in 1905. He found that nitrogen and hydrogen would combine if they were heated to a very high temperature with a very high pressure. He also found that a catalyst was needed to make the reaction occur. The catalyst he used was iron metal, though other metals are sometimes used. A catalyst is a substance used to speed up or slow down a chemical reaction. The catalyst does not undergo any change itself during the reaction.
The significance of Haber's discovery was soon apparent. World War I began in 1914. Very soon, Germany was no longer able to get nitrates from Chile. Nitrates were essential to the war effort for making explosives. But ships carrying nitrates from Chile to Germany were usually not able to get across the Atlantic Ocean.
However, nitrates can be made from ammonia using the Haber process. Germany was then able to make all the ammonia it needed. The ammonia was converted to nitrates for explosives. So a lack of nitrates from Chile did not stop the German war machine. Instead, it went on fighting for another four years.
Ammonia usually ranks about number 5 or 6 among the most highly produced chemicals in the United States. The most important use of ammonia is in synthetic fertilizers. A synthetic fertilizer is a mixture of compounds used to make plants grow better. Most farmers use huge amounts of synthetic fertilizer every year to ensure large crops.
In 1996, more than 38 million tons of nitrogen-containing synthetic fertilizer was made in the United States. Nearly half of that was anhydrous ammonia. Anhydrous means "without water." Anhydrous ammonia is simply ammonia gas. It is stored in large tanks. Farmers inject anhydrous ammonia directly into the ground to produce strong and healthy plants.
Ammonia is also found in many household cleaners, especially glass-cleaning and grease-cutting products.
The largest producer of ammonia in the world is China. Other large producers are the United States, India, Russia, Canada, Ukraine, Indonesia, and the Netherlands.
Nitrogen's role in the Oklahoma City bombing
O n April 19, 1995, a bomb exploded at the Alfred P. Murrah Federal Building in Oklahoma City, Oklahoma. The devastating impact of the explosion destroyed the building within eight seconds. Each of the nine floors collapsed on top of one another. The bomb killed 168 people and injured hundreds more. The nation was stunned to learn that the attack had not been committed by international terrorists, but by an American, Timothy McVeigh.
While searching the home of McVeigh's accomplice (a partner in a crime), Terry Nichols, investigators found a receipt for ammonium nitrate. This nitrogen compound is most commonly purchased by farmers. They use it as a fertilizer for their crops. But it can also be used as an explosive. The amount on the receipt was for 2,000 pounds. Since neither Nichols nor McVeigh was farming at the time the fertilizer was purchased, there was no real reason to purchase such a large amount of fertilizer.
Investigators determined that an ammonium nitrate bomb did, indeed, destroy the Murrah Building. The explosion resulted from a mixture of 4,800 pounds of ammonium nitrate and fuel oil from twenty plastic drums.
In June 1997, McVeigh was found guilty of all eleven charges against him, including eight counts of first-degree murder of federal agents. He was sentenced to death later that month.
Ammonia can also be converted into other forms. For example, it can be
combined with nitric acid (HNO
3
) to form ammonium nitrate (NH
4
N0
3
). And it can be combined with sulfuric acid (H
2
S0
4
) to make ammonium sulfate ((NH
4
)
2
S0
4
):
In 1996, about 2.9 million tons of ammonium nitrate and 2.6 million tons of ammonium sulfate were produced as fertilizers. These two compounds usually rank about number 14 and number 30 among chemicals produced in the United States
Ammonium nitrate and ammonium sulfate both have other uses also. For example, ammonium nitrate is used to make explosives, fireworks, insecticides and herbicides (chemicals that kill insects and weeds), and rocket fuel. Ammonium sulfate is also used in water treatment systems, as a food additive, in the tanning of leather, in fireproofing materials, and as a food additive.
Yet another important compound of nitrogen is nitric acid (HNO
3
). Nitric acid is made by reacting ammonia with oxygen:
Nitric acid usually ranks about number 13 among chemicals produced in the United States. The major use of nitric acid is to make ammonium nitrate as a synthetic fertilizer. Nitric acid is also used to make explosives, dyes, certain kinds of synthetic rubber and plastics, and in the preparation of metals.
Health effects
Nitrogen is absolutely essential to all Living organisms. It is an important part of all protein molecules. Proteins are the building material in all kinds of cells. They are also used for many other functions. For example, all living organisms use hormones to send chemical messages from one cell to another. Hormones are proteins.
Nitrogen is absolutely essential to all living organisms. It is an important part of all protein molecules.
Nitrogen is also used to make nucleic acids. Nucleic acids have many important functions in living organiams. For one thing, they store the organism's genetic information. The genetic information is the set of instructions that tell every cell what its job in the organism is. It passes on that information from one generation to the next.
In this statement I don't understand how 3/4 of nitrogen is compound of this element.