SULFUR

Overview
Sulfur belongs to the chalcogen family. Other members of the family are oxygen, selenium, tellurium, and polonium. These elements make up Group 16 (VIA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to each other.
The term chalcogen comes from two Greek words meaning "ore forming." An ore is a naturally occurring mineral used as a source for an element. Many ores are compounds of a metal and oxygen or a metal and sulfur. Compounds that contain two elements, one of which is sulfur, are called sulfides. For example, a beautiful gold-colored mineral is called pyrite, or "fool's gold," because it looks so much like real gold. Pyrite is iron sulfide (FeS 2 ).
Sulfur was known to ancient peoples. Its physical and chemical properties are very distinctive. It often occurs as a brilliant yellow powder. When it burns, it produces a clear blue flame and a very strong odor.
SYMBOL
S
ATOMIC NUMBER
16
ATOMIC MASS
32.064
FAMILY
Group 16 (VIA)
Chalcogen
PRONUNCIATION
SUL-fur
Sulfur, also spelled as sulphur, is a very important element in today's world. Its most important use is in the manufacture of sulfuric acid (H 2 SO 4 ). There is more sulfuric acid made than any other chemical in the world. It has an enormous number of important uses.
Discovery and naming
Sulfur must have been well known to ancient peoples. They sometimes referred to it as brimstone. Sulfur sometimes occurs in bright yellow layers on the top of the earth. It has a sharp, offensive odor. When it burns, it gives off a strong, suffocating smell. The odor is like that produced when a match is struck.
The Bible mentions brimstone in a number of places. For example, Sodom and Gomorrah were two towns destroyed by God for the wicked ways of their citizens: "The Lord rained upon Sodom and upon Gomorrah brimstone and fire."
But ancient people certainly did not think about sulfur the way modern chemists do. In fact, they used the word "element" to talk about anything that was basic. Ancient Greek philosophers, for example, thought that everything consisted of four elements: earth, fire, water, and air. Other philosophers thought there were only two elements: sulfur and mercury.
But early thinkers were often confused as to what they meant by the word "sulfur." They often were talking about anything that burned and gave off large amounts of smoke. To them, "sulfur" was really a "burning substance." It took centuries for scientists to identify sulfur as an element.
Physical properties
Sulfur exists in two allotropic forms. Allotropes are forms of an element with different physical and chemical properties. The two forms of sulfur are known as α-form and β-form (the Greek letters alpha and beta, respectively). Both allotropes are yellow, with the α-form a brighter yellow and the β-form a paler, whitish-yellow. The α-form changes to the β-form at about 94.5°C (202°F). The α-form can be melted at 112.8°C (235.0°F) if it is heated quickly. The β-form has a melting point of 119°C (246°F). The boiling point of the α-form is 444.6°C (832.3°F).
The two allotropes have densities of 2.06 grams per cubic centimeter (α-form) and 1.96 grams per cubic centimeter (β-form). Neither allotrope will dissolve in water. Both are soluble
Another allotrope of sulfur is formed when the element is melted. This allotrope has no crystalline shape. It looks like a dark brown, thick, melted plastic.
Chemical properties
Sulfur's most prominent chemical property is that it burns. When it does so, it gives off a pale blue flame and sulfur dioxide (SO 2 ) gas. Sulfur dioxide has a very obvious strong, choking odor.
Sulfur sometimes occurs in bright yellow layers on the top of the earth. It has a sharp, offensive odor.
Sulfur also combines with most other elements. Sometimes it combines with
them easily at room temperature. In other cases, it must be heated. The
reaction between
magnesium
and sulfur is typical. When the two elements are heated, they combine to
form magnesium sulfide (MgS):

Sulfur also combines with
hydrogen
gas:
The compound formed in this reaction is hydrogen sulfide (H 2 S). Hydrogen sulfide has one of the best known odors of all compounds. It smells like rotten eggs. Hydrogen sulfide is added to natural gas (methane) used in homes for cooking and heating. Methane is odorless. So the unique smell of hydrogen sulfide makes it easy to know when there is a methane leak.
Occurrence in nature
At one time, sulfur occurred in layers along the Earth's surface. They were easy for humans to find and take. Deposits like these are more difficult to find today. One place they still occur is in the vicinity of volcanoes. Sulfur is released from volcanoes as a gas. When it reaches the cold air, it changes back to a solid. It forms beautiful yellow deposits along the edge of a volcano.
Large supplies of sulfur still occur underground. They are removed by the Frasch process (see accompanying sidebar).
Sulfur also occurs in a number of important minerals. Some examples are barite, or barium sulfate (BaSO 4 ); celestite, or strontium sulfate (SrSO 4 ); cinnabar, or mercury sulfide (HgS); galena, or lead sulfide (PbS); pyrites, or iron sulfide (FeS 2 ); sphalerite, or zinc sulfide (ZnS); and stibnite, or antimony sulfide (Sb 2 S 3 ).
The abundance of sulfur in the Earth's crust is thought to be about 0.05 percent. It ranks about number 16 among the elements in terms of their abundance in the earth. It is more abundant than carbon, but less abundant than barium or strontium.
The largest producers of sulfur in the world are the United States, Canada, China, Russia, Mexico, and Japan. In 1996, the United States produced about 11,800,000 metric tons of sulfur. It is mined in 30 states, Puerto Rico, and the U.S. Virgin Islands.
Isotopes
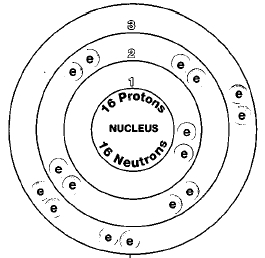
There are four naturally occurring isotopes of sulfur: sulfur-32, sulfur-33, sulfur-34, and sulfur-36. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Sulfur occurs in the vicinity of volcanoes.
Six radioactive isotopes of sulfur are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
One radioactive isotope of sulfur, sulfur-35, is used commercially. In medicine, the isotope is used to study the way fluids occur inside the body. It also has applications in research as a tracer. A tracer is a radioactive isotope whose presence in a system can easily be detected. The isotope is injected into the system at some point. Inside the system, the isotope gives off radiation. That radiation can be followed by means of detectors placed around the system.
As an example, a company that makes rubber tires might want to know what happens to the sulfur added to tires. Sulfur-35 is added to rubber along with non-radioactive sulfur. Researchers follow the radioactive isotope in the tires to see what happens to the sulfur when the tires are used.
The Frasch method of removing sulfur
T he Frasch method is one of the most famous mining systems ever invented. It was developed by German-American chemist Herman Frasch (1851-1914) in 1887.
The Frasch method is based on the low melting point of sulfur. The element melts at a temperature slightly higher than that of boiling water (100°C). Here is how the method works:
A set of three nested pipes (one inside each other) is sunk into the ground. The innermost pipe has a diameter of about an inch. The middle pipe has a diameter of about four inches. And the outer pipe has a diameter of about eight inches.
A stream of superheated water is injected into the outer pipe. Superheated water is water that is hotter than its boiling point, but that has not started to boil. Superheated water can be made by raising the pressure on the water. Its temperature can reach 160°C (320°F).
The superheated water passes down the outer pipe into the underground sulfur, causing it to melt. The molten (melted) sulfur forms a lake at the bottom of the pipe.
At the same time, a stream of hot air under pressure is forced down the innermost (one-inch) pipe. The hot air stirs up the molten sulfur and hot water at the bottom of the pipe. A foamy, soupy mixture of sulfur and water is formed. The mixture is forced upward through the middle pipe. When it reaches the surface, it is collected. The sulfur cools and separates from the water.
Similar applications of sulfur-35 involve studying sulfur in steel when it is made, seeing how sulfur affects the way engines operate, following what happens when proteins (which contain sulfur) are digested, and learning how drugs that contain sulfur are processed in the body.
Extraction
Like coal, sulfur sometimes occurs in thick layers under ground. One way to remove sulfur would be to mine it the way coal is mined. But a much easier method for removing sulfur from the ground is the Frasch method (see accompanying sidebar).
Uses
Sulfur has relatively few uses as an element. One of the most important of those uses is in vulcanization. Vulcanization is the process of adding sulfur to rubber to make it stiff and hard. It keeps the rubber from melting as it gets warmer. The discovery of vulcanization by Charles Goodyear (1800-60) in 1839 is one of the greatest industrial accomplishments of modern times.
Some powdered sulfur is also used as an insecticide. It can be spread on plants to kill or drive away insects that feed on the plants. By far the majority of sulfur is used, however, to make sulfur compounds. The most important of these is sulfuric acid (H 2 SO 4 ).
Compounds
Nearly 90 percent of all sulfur produced goes into sulfuric acid. Sulfuric acid is the number one chemical in the world in terms of the amount produced. Each year, almost twice as much sulfuric acid is made as the next highest chemical, nitrogen. In 1996, more than 45 million tons of sulfuric acid were produced in the United States alone.
The greatest portion, nearly 75 percent, of sulfuric acid is used to make fertilizers. The next most important use, 10 percent, is in the petroleum industry. Other important uses of sulfuric acid are in the treatment of copper ores; the production of paper and paper products; the manufacture of other agricultural chemicals; and the production of plastics, synthetic rubber, and other synthetic materials.
Vulcanization is the process of adding sulfur to rubber to make it stiff and hard.
Sulfuric acid is also used in smaller amounts to make explosives, water treatment chemicals, storage batteries, pesticides,
Health effects
The cleansing power of sulfur has been known for many centuries. At one time, ancient physicians burned sulfur in a house to cleanse it of impurities. Creams made with sulfur were used to treat infections and diseases. In fact, sulfur is still used to treat certain medical problems. Sulfur is prepared in one of three forms. Precipitated sulfur (milk of sulfur) is made by boiling sulfur with lime. Sublimed sulfur (flowers of sulfur) is pure sulfur powder. And washed sulfur is sulfur treated with ammonia water. Washed sulfur is used to kill parasites (organisms that live on other organisms) such as fleas and ticks. It is also used as a laxative, a substance that helps loosen the bowels.
Sulfur is a macronutrient for both plants and animals. A macronutrient is an element needed in relatively large amounts to insure the good health of an organism. Sulfur is used to make proteins and nucleic acids, such as DNA. It also occurs in many essential enzymes. Enzymes are chemicals that make chemical reactions occur more quickly in cells. Humans usually have no problem getting enough sulfur in their diets. Eggs and meats are especially rich in sulfur.
A person who does not get enough sulfur in his or her diet develops certain health problems. These include itchy and flaking skin and improper development of hair and nails. Under very unusual conditions, a lack of sulfur can lead to death. Such conditions would be very rare, however.
The cleansing power of sulfur has been known for many centuries.
Plants require sulfur for normal growth and development. When plants do not get enough sulfur from the soil, their young leaves start to turn yellow. Eventually, this yellowing extends to the whole plant. The plant may develop other diseases as a result.
May I ask what form of sulfur is used in extemporaneously compounding lotions?
TIA