PHOSPHORUS

Overview
Phosphorus is found in Group 15 (VA) of the periodic table. The periodic table is a chart that that shows how chemical elements are related to each other. Phosphorus is part of the nitrogen family along with nitrogen, arsenic, antimony, and bismuth.
Phosphorus was first discovered in 1669 by German physician Hennig Brand (ca. 1630-92). Brand is somewhat famous in chemistry. He is sometimes called the last of the alchemists. Alchemy was a kind of pre-science that existed from about 500 B.C. to about the end of the 16th century. Alchemists wanted to find a way of changing lead, iron, and other metals into gold. They also wanted to find a way of having eternal life. Alchemy contained too much magic and mysticism to be a real science. But it developed a number of techniques and produced many new materials that were later found to be useful in modern chemistry.
SYMBOL
P
ATOMIC NUMBER
15
ATOMIC MASS
30.97376
FAMILY
Group 15 (VA)
Nitrogen
PRONUNCIATION
FOS-fer-us
Brand was convinced that the key to changing metals into gold could be found in urine. He decided to look for the "magic substance" that could change lead into gold in urine. In the process of heating and purifying urine, he obtained phosphorus. The discovery was important because it was the first time someone had discovered an element not known to ancient peoples. In that regard, Brand was the first person who could be called the discoverer of an element.
Phosphorus is a fascinating element that occurs in at least three very different forms. If left exposed to the air, it catches fire on its own. It also glows in the dark. Today, its most important use is in the manufacture of phosphoric acid (H 3 PO 4 ). Phosphoric acid, in turn, is used to manufacture fertilizers and a number of other less important products.
Discovery and naming
Phosphorus and its compounds may have been known before Brand's discovery. Old manuscripts refer to materials that glow in the dark. The word used for such materials today is phosphorescent. Early Christians noted the use of "perpetual lamps" that glowed in the dark. The lamps may have contained phosphorus or one of its compounds.
Still, Brand was the first to record the process of making pure phosphorus. No one knows how he decided that urine might contain a chemical that could be used to turn lead into gold. His experiments to find such a chemical were, of course, a failure. But he made an accidental discovery along the way. That discovery was a material that glowed in the dark: phosphorus.
Scientists were fascinated when they heard of Brand's discovery. They tried to repeat his research. Some tried to talk him into selling his discovery to kings and princes. The new element seemed to be a way of getting rich and becoming famous.
But Brand was never interested in these ideas. Instead, he gave away all of the phosphorus he prepared. Other scientists soon began to experiment with the element. One of the first discoveries they made was how dangerous phosphorus is. One scientist wrote that a servant left some phosphorus on top of his bed one day. Later that night, the bed covers burst into flame. The phosphorus had caught fire by itself!
Eventually, Brand's method of making phosphorus became widely known. The element joined iron, gold, silver, arsenic, and the handful of other elements known to early chemists.
Little is known about what happened to Brand after his discovery. In fact, there is no record of where or when he died.
Physical properties
Phosphorus exists in at least three allotropic forms. Allotropes are forms of an element with different physical and chemical properties. The three main allotropes are named for their colors: white phosphorus (also called yellow phosphorus), red phosphorus, and black phosphorus (also called violet phosphorus). These allotropes all have different physical and chemical properties.
White phosphorus is a waxy, transparent solid. Its melting point is 44.1°C (111°F) and its boiling point is 280°C (536°F). It has a density of 1.88 grams per cubic centimeter. If kept in a vacuum, it sublimes if exposed to light. Sublimation is the process by which a solid changes directly to a gas when heated, without first changing to a liquid. White phosphorus is phosphorescent. It gives off a beautiful greenish-white glow. It does not dissolve well in water, although it does dissolve in other liquids, such as benzene, chloroform, and carbon disulfide. White phosphorus sometimes appears slightly yellowish because of traces of red phosphorus.
Red phosphorus is a red powder. It can be made by heating white phosphorus with a catalyst to 240°C (464°F). A catalyst is a substance used to speed up or slow down a chemical reaction without undergoing any change itself. Without a catalyst, red phosphorus sublimes at 416°C (781°F). Its density is 2.34 grams per cubic centimeter. It does not dissolve in most liquids.
Black phosphorus looks like graphite powder. Graphite is a form of carbon used in "lead" pencils. Black phosphorus can be made by applying extreme pressure to white phosphorus. It has a density of 3.56 to 3.83 grams per cubic centimeter. One of its interesting properties is that it conducts an electric current in spite of being a non-metal.
Brand was convinced that the key to changing metals into gold could be found in urine. Instead, he found phosphorus.
Chemical properties
White phosphorus is the form that occurs most commonly at room temperatures. It is very reactive. It combines with oxygen so easily that it catches fire spontaneously (automatically). As a safety precaution, white phosphorus is stored under water in chemical laboratories.

Phosphorus combines easily with the halogens. The halogens are the
elements that make up Group 17 (VIIA) of the periodic table. They include
fluorine, chlorine, bromine, iodine,
and
astatine
. For example, the reaction between phosphorus and chlorine is:
Phosphorus also combines with metals to form compounds known as
phosphides:
White phosphorus combines with oxygen so easily that it catches fire automatically. As a safety precaution, white phosphorus is stored under water in chemical laboratories.
Occurrence in nature
The abundance of phosphorus in the Earth's crust is estimated to be 0.12 percent, making it the 11th most common element. It usually occurs as a phosphate. A phosphate is a compound that contains phosphorus, oxygen, and at least one more element. An example is calcium phosphate, Ca 3 (PO 4 ) 2 .
The only important commercial source of phosphorus is phosphate rock. Phosphate rock is primarily calcium phosphate. The United States is the largest producer of phosphate rock in the world. In 1996, 13,300,000 metric tons of phosphate rock were mined in the United States. That amounted to about a third of the world's total phosphate rock.
About 86 percent of phosphate rock comes from North Carolina and Florida. Smaller amounts are also mined in Idaho and Utah. Other major producers of phosphate rock are Morocco, China, Russia, Tunisia, Jordan, and Israel.
Isotopes
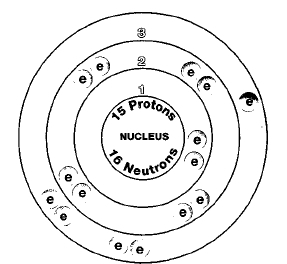
Only one naturally occurring isotope of phosphorus exists, phosphorus-31. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Six radioactive isotopes of phosphorus are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
One radioactive isotope, phosphorus-32, has applications in medicine, industry, and tracer studies. A tracer is a radioactive isotope whose presence in a system can easily be detected. The isotope is injected into the system where it gives off radiation. The radiation is followed by means of detectors placed around the system.
Phosphorus-32 is especially useful in medical studies, because phosphorus occurs in many parts of the body. Radioactive phosphorus can be used as a tracer to study parts of the body as well as chemical changes inside the body. Radioactive phosphorus can also determine how much blood is in a person's body. It can also help locate the presence of tumors in the brain, eyes, breasts, and skin. Finally, it is sometimes used to treat certain
Phosphorus-32 is important in a variety of scientific studies. For example, it is added to tires when they are made. Then, the radiation it gives off can be studied as the tires are used. This information tells where the tire wears out and how long it takes to wear out.
Extraction
It is possible to make pure phosphorus from phosphate rock. The rock is
mixed with sand and coke (pure carbon). The mixture is then heated in an
electric furnace. An electric furnace is a device for producing very high
temperatures. Pure phosphorus is produced in this reaction. It escapes
from the mixture as a vapor (gas). The cooled vapor solidifies into white
phosphorus. The reaction is:
Radioactive phosphorus helps locate the presence of tumors in the brain, eyes, breasts, and skin.
This reaction is not very important because pure phosphorus has few uses. The most important compounds of phosphorus are all made from phosphate rock or calcium phosphate. Therefore, the most important step in producing "phosphorus" is
Uses and compounds
In 1996, 91 percent of all the phosphate rock mined in the United States was used to make fertilizer. Modern farmers use enormous amounts of synthetic (artificial) fertilizer on their crops. This synthetic fertilizer contains nitrogen, phosphorus, and potassium, the three elements critical to growing plants. These elements normally occur in the soil, but may not be present in large enough amounts. Adding them by means of synthetic fertilizer helps plants grow better. Most farmers add some form of synthetic fertilizer to their fields every year. This demand for synthetic fertilizers accounts for the major use of phosphorus compounds.
Phosphorus and its compounds have other uses. These uses account for about 10 percent of all the phosphorus produced. For example, the compounds known as phosphorus pentasulfide (P 2 S 5 ) and phosphorus sesquisulfide (P 4 S 3 ) are used to make ordinary wood and paper safety matches. These compounds coat the tip of the match. When the match is scratched on a surface, the phosphorus pentasulfide or phosphorus sesquisulfide bursts into flame. It ignites other chemicals on the head of the match.
Another compound of phosphorus with a number of uses is phosphorus oxychloride (POCl 3 ). This compound is used in the manufacture of gasoline additives, in the production of certain kinds of plastics, as a fire retardant agent, and in the manufacture of transistors for electronic devices.
Health effects
Phosphorus is essential to the health of plants and animals. Many essential chemicals in living cells contain phosphorus. One of the most important of these chemicals is adenosine triphosphate (ATP). ATP provides the energy to cells they need to stay alive and carry out all the tasks they have to perform. Phosphorus is critical to the development of bones and teeth. Nucleic acids also contain phosphorus. Nucleic acids are chemicals that perform many functions in living organisms. For example, they carry the genetic information in a cell. They tell the cell what chemicals it must make. It also acts as the "director" in the formation of those chemicals.
The daily recommended amount of phosphorus for humans is one gram. It is fairly easy to get that much phosphorus every day through meat, milk, beans, and grains.
Two phosphorus compounds are used to coat the tip of a match.
On the other hand, elemental phosphorus is extremely dangerous. Elemental phosphorus is phosphorus as an element, not combined with other elements. Swallowing even a speck of white phosphorus produces severe diarrhea with loss of blood; damage to the liver, stomach, intestines, and circulatory system (blood flow system); and coma. Swallowing a piece of white phosphorus no larger than 50 to 100 milligrams (0.0035 ounce) can even cause death.
Handling white phosphorus is dangerous as well. It causes serious skin burns.
Making lakes too healthy
T he second most important use of phosphate compounds is in making detergents. The compound most often used in detergents is called sodium tripolyphosphate, or STPP (Na 5 P 3 O 10 ).
STPP adds a number of benefits to a detergent. For example, it can kill some bacteria and prevent washers from becoming corroded (rusted) and clogged. The most important function in detergents, however, is as a water-softening agent.
Natural water often contains chemicals that keep soaps and detergents from sudsing. They reduce the ability of soaps and detergents to clean clothes. STPP has the ability to capture these chemicals. It greatly improves the ability of soaps and detergents to make suds and clean clothes. The first detergent to use STPP was Tide, in 1947. The introduction of Tide brought about a revolution in clothes cleaning.
But STPP can create problems for the environment. After detergents have been used, they often end up in rivers and streams and, eventually, in lakes from waste water. And that's just fine for the algae that live in those lakes. Algae are tiny green plants that use phosphorus as they grow. As more detergents get into lakes, the amount of STPP increases. That means there is more phosphorus in a lake and that, in turn, means that algae begin to grow much faster.
In some cases, there is so much STPP and phosphorus in a lake that algae grow out of control, clogging the lake with algae and other green plants. The lake slowly turns into a swamp, and finally into a meadow. The lake disappears!
Many people became concerned about this problem in the 1960s. They demanded that less STPP be used in detergents. A number of cities and states banned the sale of STPP detergents. STPP production had grown rapidly from 1.10 billion pounds in 1955 to 2.44 billion pounds in 1970. But then production began to drop off. By the mid-1990s, production had dropped well below a billion pounds a year.
Interestingly, red phosphorus does not have the same effects. It is considered to be relatively safe. It is dangerous only if it contains white phosphorus mixed with it.
I need some explanation pliz