PLATINUM

Overview
Platinum is a transition metal in Group 10 (VIIIB) of the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. Platinum is also a member of a group of metals named after itself. Other platinum metals include ruthenium, rhodium, palladium, osmium, and indium. They are found in Rows 5 and 6 of Groups 8 through 10 in the periodic table. Platinum is also considered to be a precious metal. A precious metal is one that is rare and desirable.
The platinum group metals are sometimes referred to as the noble metals. That term comes from the fact that they are all relatively inactive. They do not combine with or interact with most other elements or compounds. This chemical inactivity accounts for some of the uses of the platinum metals. For example, platinum is often used to make laboratory equipment because it will not react with materials that come into contact with the equipment.
SYMBOL
Pt
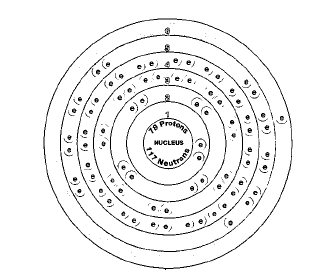
ATOMIC NUMBER
78
ATOMIC MASS
195.08
FAMILY
Group 10 (VIIIB)
Transition metal;
platinum group
PRONUNCIATIONY
PLAT-num
The primary use of platinum and other platinum metals is as catalysts. A catalyst is a substance used to speed up or slow down a chemical reaction without undergoing any change itself. For example, the catalytic converter in an automobile's exhaust system may contain a platinum metal.
Discovery and naming
The first known reference to platinum can be found in the writings of Italian physician, scholar, and poet Julius Caesar Scaliger (1484-1558). Scaliger apparently saw platinum while visiting Central America in 1557. He referred to a hard metal that the natives had learned to work with, but the Spanish had not. The metal had been called platina ("little silver") by the natives. The name was given to the material because it got in the way of mining silver and gold . Since the natives knew of no use for the platina, they thought of it as a nuisance.
The first complete description of platinum was given by the Spanish military leader Don Antonio de Ulloa (1716-95). While serving in South America from 1735 to 1746, de Ulloa collected samples of platinum. He later wrote a report about the metal, describing how it was mined and used. De Ulloa is often given credit for discovering platinum on the basis of the report he wrote.
Reports of the new element spread through Europe. Scientists were fascinated by its physical properties. It was not only beautiful to look at, but resistant to corrosion (rusting). Many people saw that it could be used in jewelry and art objects, as with gold and silver. Demand for the metal began to grow, leading to what was then called the "Platinum Age in Spain."
Physical properties
Platinum is a silver-gray, shiny metal that is both malleable and ductile. Malleable means capable of being hammered into thin sheets. Platinum can be hammered into a fine sheet no more than 100 atoms thick, thinner than aluminum foil.
Ductile means the metal can be drawn into thin wires. Platinum has a melting point of about 1,773°C (3,223°F) and a boiling point of about 3,827°C (6,921°F). Its density is 21.45 grams per cubic centimeter, making it one of the densest elements.
Chemical properties
Platinum is a relatively inactive metal. When exposed to air, it does not tarnish or corrode. It is not attacked by most acids, but will dissolve in aqua regia. Aqua regia is a mixture of

An unusual property of platinum is that it will absorb large quantities of hydrogen gas at high temperatures. The platinum soaks up hydrogen the way a sponge soaks up water.
Occurrence in nature
The platinum metals are often found together in nature. In fact, one of the problems in producing platinum is finding a way of separating it from the other platinum metals. Unlike gold, however, these metals do not occur in masses large enough to mine. Instead, they are usually obtained as byproducts from mining other metals, such as copper and nickel.
Platinum is one of the rarest elements. Its abundance is estimated to be about 0.01 parts per million in the Earth's crust. The world's largest supplier of platinum by far is South Africa. In 1996, that nation produced 117,000 kilograms of platinum. The next largest producer was Canada, producing only 8,260 kilograms in 1996. The only other large producer of platinum is the United States. Most of the platinum in the United States comes from the Stillwater Mine in Montana.
Isotopes
Six naturally occurring isotopes of platinum exist: platinum-190, platinum-192, platinum-194, platinum-195, platinum-196, and platinum-198. Of these, only platinum-190 is radioactive. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Artificially radioactive isotopes of platinum have also been produced. These isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
No radioactive isotope of platinum has any commercial application.
Extraction
The major challenge in obtaining pure platinum is separating it from other platinum metals. The first step in this process is to dissolve the mixture in aqua regia. Platinum dissolves in aqua regia, and other platinum metals do not. Platinum metal can then be removed from the aqua regia in a form known as platinum sponge. Platinum sponge is a sponge-like material of black platinum powder. Finally, the powder is heated to very high temperatures and melted to produce the pure metal.
Uses
If asked, most people would probably name jewelry as the most important use of platinum. And the metal is used for that purpose. It is hard, beautiful, corrosion-resistant—ideal for making bracelets, earrings, pins, watch bands, and other types of jewelry.
However, jewelry is not the most important use of platinum. The making of catalysts is. For example, platinum catalysts are
Platinum catalysts are also used to make compounds that end up as fertilizers, plastics, synthetic fibers, drugs and pharmaceuticals, and dozens of other everyday products. For example, platinum is used in the manufacture of nitric acid (HNO 3 ).
Nitric acid is used to produce ammonia, which, in turn, is used to make fertilizers.
Probably the best known use of platinum as a catalyst is in cars. All new automobiles have a catalytic convertor in the exhaust system. A catalytic converter is a device that helps gasoline burn more completely. It reduces the amount of pollutants released to the air. Most catalytic converters contain platinum or other platinum metals.
Platinum is used in other parts of a car or truck. Certain types of spark plugs, for example, may contain platinum. Overall, the greatest single use of platinum in the United States is in the manufacture of automobiles and trucks.
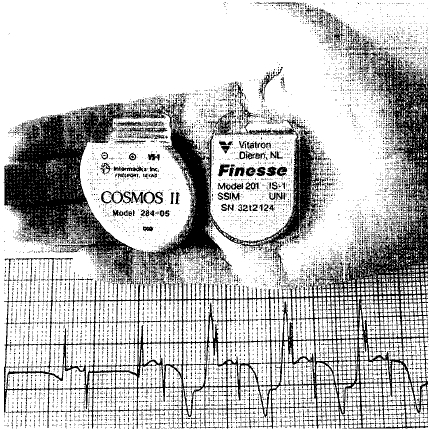
Many uses of platinum depend on its chemical inactivity. For example, some people have to have artificial heart pacemakers implanted into their chests. An artificial pacemaker is a device that makes sure the heart beats in a regular pattern. It usually replaces a body part that performs that function but has been damaged. Artificial pacemakers are usually made out of platinum. The platinum protects the pacemaker from corroding or being destroyed by adds inside the body.
Platinum is also used in small amounts in alloys. For example, cobalt alloyed with platinum makes a powerful magnet. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals. The platinum-cobalt magnet is one of the strongest magnets known.
Compounds
Relatively few platinum compounds are commercially important.
Artificial pacemakers are usually made out of platinum. The platinum protects the pacemaker from corroding or being destroyed by acids inside the body.
Health effects
Platinum dust and some platinum compounds can have mild health effects. If inhaled, they can cause sneezing, irritation of the nose, and shortness of breath. If spilled on the skin, they can cause a rash and skin irritation.