GADOLINIUM
Overview
Gadolinium was named for Finnish chemist Johan Gadolin (1760-1852). Gadolin served for many years as professor of chemistry at the University of Åbo in Finland. He was the first person to study an unusual black stone discovered near the town of Ytterby, Sweden, in 1787. The stone was an unusually important discovery. Chemists isolated nine new elements from the stone, one of which was gadolinium.
SYMBOL
Gd
ATOMIC NUMBER
64
ATOMIC MASS
157.25
FAMILY
Lanthanide
rare earth metal)
PRONUNCIATION
gad-uh-LIN-ee-um
Gadolinium is in Row 6 of the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. The elements in Row 6 are called rare earth elements. They really aren't rare, but they are difficult to separate. For this reason, scientists know less about the rare earth elements than they do about most other elements. The rare earth metals are also called lanthanides. That name comes from the first element in Row 6, lanthanum.
Discovery and naming
Two unusual rocks were discovered in Sweden near the end of the eighteenth century. The rocks were unusual because they both contained a complex mixture of substances. Chemists worked for nearly a century to separate the mixtures and find out what they were. All fifteen rare earth elements were first discovered in the two Swedish rocks.
One rock contained a mineral that had never been seen before, cerite. Cerite was first discovered in 1803. The last new element found in cerite was not identified until almost a century later, in 1901. In 1880, French chemist Jean-Charles Galissard de Marignac (1817-94) was studying a new material found in cerite called samaria. Earlier chemists had identified samaria as a new element.
Marignac found that samaria was not a pure element. Instead, it consisted of two parts, which he called samaria and gadolinia. He believed each was a new element. He was right about gadolinia, but wrong about samaria.
Physical properties
Gadolinium has a shiny metallic luster with a slight yellowish tint. It is both ductile and malleable. Ductile means capable of being made into wires. Malleable means capable of being hammered or rolled into thin sheets. It has a melting point of 1,312°C (2,394°F) and a boiling point of about 3,000°C (5,400°F). Its density is 7.87 grams per cubic centimeter.
Few elements are as strongly magnetic as gadolinium. It also has the highest neutron-absorbing ability of any element. A piece of gadolinium stops neutrons better than any other element.
Chemical properties
Gadolinium metal is not especially reactive. It dissolves in acids and reacts slowly with cold water. It also reacts with oxygen at high temperatures.
Occurrence in nature
The abundance of gadolinium in the Earth's surface is estimated at about 4.5 to 6.4 parts per million. That would make it one of the most abundant of the rare earth elements. It ranks above bromine and uranium, but just below lead and boron in order of abundance. Some minerals in which it occurs are monazite, bastnasite, samarskite, gadolinite, and xenotime.
Isotopes
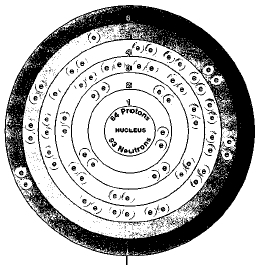
Seven naturally occurring isotopes of gadolinium are known. They are gadolinium-152, gadolinium-154, gadolinium-155, gadolinium-156, gadolinium-157, gadolinium-158, and gadolinium-160. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope. One of gadolinium's naturally occurring isotopes is radioactive—gadolinium-152. A radioactive isotope is one that breaks apart and gives off some form of radiation.
Radioactive isotopes can also be produced artificially when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive. At least eleven artificial radioactive isotopes have been produced. Some of these are used in medicine. For example, gadolinium-153 is used to study the composition of bones. The radiation it gives off acts like X rays, penetrating the bones to reveal the minerals present.
Gadolinium is also used in another X-ray-like technique called neutron radiography. In this technique, neutrons are fired through a sample of material. The neutrons act somewhat like X rays. They show the structure of the material. Adding gadolinium to the back side of the material makes the neutron image easier to read. Neutron radiography is especially useful because one can look for damage inside a piece of metal without having to take the material apart.
Extraction
The method for obtaining gadolinium from its ores is similar to that for
other rare earth elements. The ore is converted into gadolinium chloride
(GdCl
3
) or gadolinium fluoride (GdF
3
). Passing an electric current through the first compound releases pure
gadolinium:
Nine new elements, including gadolinium, came from an unusual black stone found in Ytterby, Sweden, in 1787.
Adding calcium to the second compound also releases pure gadolinium:
Uses and compounds
Gadolinium is used in control rods in nuclear power plants. Energy produced during nuclear fission is used to generate electricity. Nuclear fission is the process in which large atoms (usually uranium or plutonium ) break apart, releasing energy. The smaller atoms produced are called fission products and are radioactive.
Neutrons are also produced in the reaction. In order for a nuclear power plant to work properly, the number of neutrons must be carefully controlled. Rods containing gadolinium are raised out of or lowered into the reactor. This allows more or fewer neutrons to remain in the reaction.
Gadolinium also has medical uses. It is used to locate the presence of tumors in the inner ear. Gadolinium is injected into the blood stream. It then goes to any tumor that happens to be present in the ear. The tumor appears darker when seen with X rays.
Gadolinium is used to locate tumors in the inner ear.
Gadolinium compounds are used as phosphors in television tubes. A phosphor is a material that shines when struck by electrons. The color of the phosphor depends on the elements of which it is made.
Gadolinium is also found in alloys and special minerals known as yttrium garnets. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals. Gadolinium alloys are easier to work with than alloys without gadolinium. Gadolinium yttrium garnets are used in microwave ovens to produce the microwaves.
What on Earth is an "Earth"?
B ooks on the chemical elements sometimes talk about gadolin ium and gadoli nia; about erb ium and sometimes erb ia; sometimes about samar ium and sometimes samar ia. Is the -ium ending any different from the -ia ending?
The answer is yes. Almost all metal names end in -ium or just -um, like sod ium, potass ium, magnes ium, alumin um, and gadolin ium. The ending -ia or just -a, on the other hand, stands for the form in which an element occurs in the earth. When miners take gadolinium out of the earth, it is called gadolinia.
The natural form of the element is often called an "earth." Gadolinium is the element that comes from the earth, gadolinia. Earths are compounds of the element and one or more other element. Two common combining elements are oxygen and sulfur. For example, gadolinia contains gadolinium oxide (Gd 2 O 3 ).
These terms can be confusing when reading the history of chemical elements. Many elements were first discovered not in their pure form, but as compounds—as earths.
Health effects
Not many details of the health effects of gadolinium are known. It is usually handled as if it were very toxic.