FRANCIUM

Overview
Francium is an alkali metal, a member of Group 1 (IA) in the periodic table. The periodic table is a chart that shows how chemical elements are related to each other.
Francium may be the rarest element found on the Earth's surface. Some experts believe that no more than 15 grams (less than an ounce) of the element exists in the Earth's crust. The element was discovered in 1939 by French chemist Marguerite Perey (1909-975). All isotopes of francium are radioactive.
Discovery and naming
Francium was one of the last naturally-occurring elements to be discovered. Chemists had been searching for it since the development of the periodic table.
In the early 1900s, nearly all boxes on the periodic table had been filled. One element had been found to fit into each box. By the 1930s, only three remained empty—elements with atomic numbers of 43, 85, and 87.
SYMBOL
Fr
ATOMIC NUMBER
87
ATOMIC MASS
223.0197
FAMILY
Group 1 (IA)
Alkali metal
PRONUNCIATION
FRAN-see-um
This search produced a number of incorrect results. For example, American chemist Fred Allison (1882-1974) announced the discovery of elements 85 and 87 in 1931. He suggested the names of alabamine and virginium, in honor of the states in which he was born (Virginia) and where he worked (Alabama). But other scientists were not able to confirm Allison's discoveries.
Element 87 was isolated by Perey, who was studying the radioactive decay of the element actinium while working at the Curie Institute in Paris, France. Radioactive elements like actinium break apart spontaneously, giving off energy and particles. This process results in the formation of simpler new elements.
Perey found that 99 percent of all actinium atoms decay into thorium . The remaining one percent breaks down into a new element, number 87. She suggested the name francium, in honor of her homeland, France.
Physical and chemical properties
Until very recently, there was not enough francium to permit a study of its properties. In 1991, scientists confirmed that the element was similar to the other alkali metals above it on the periodic table. The alkali metals are the elements in Group 1.
Occurrence in nature
Francium is now produced in particle accelerators. A particle accelerator is also called an atom smasher. This machine accelerates small particles, like protons, to nearly the speed of light, 300,000,000 kilometers per second (186,000 miles per second). The particles collide with target atoms, such as copper, gold , or tin . Target atoms fragment, forming new elements and particles.
The most important work on francium is being conducted at the State University of New York at Stony Brook. Scientists there have found a way to trap a collection of francium atoms in the middle of a magnetic field. They can be held there long enough for scientists to perform measurements.
Francium may be the rarest element found on the Earth's surface. Some experts believe that no more than 15 grams (less than an ounce) of the element exists in the Earth's crust.
Isotopes
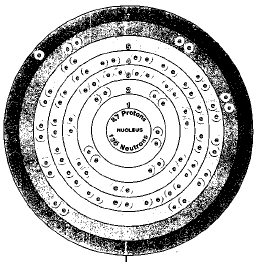
Nearly twenty isotopes of francium have been found. The most stable is francium-223. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Marguerite Perey | French physicist

M arguerite Perey (1909-75) was a French physicist who discovered the element francium. Perey was interested in science even as a small child. However, her father died early on, and there was no money for Perey to attend a university. Instead, she found a job at the Radium Institute in Paris. The Radium Institute had been founded by Marie Curie (1867-1934) and her husband, Pierre Curie (1859-1906), to study radioactive materials.
Perey was originally hired for a three-month period. But Madame Curie was very impressed with Perey's skills in the laboratory. Perey eventually ended up working at the Radium Institute until 1935.
One of the projects Perey worked on was the radioactive decay of actinium. When actinium decays, it gives off radiation and changes into another element, thorium. Thorium, in turn, also gives off radiation and changes into another element, radium. This process is repeated a number of times. In each step, a radioactive element decays to form another element.
As Perey studied this series of reactions, she made an interesting discovery. The mixture of elements that are formed in these reactions contained a substance she did not recognize. She decided to find out what that substance was. She was eventually able to show that it was a new element, with atomic number 87. The element was one of the last naturally occurring elements to be discovered. Perey named the element in honor of her native land, France.
Perey was the first woman ever elected to the French Academy of Science. Even Marie Curie had not earned that honor. Perey died in 1975 after a 15-year-long battle with cancer.
Francium-223 has a half life of 22 minutes. The half life of a radioactive element is the time it takes for half of a sample of the element to break down. That means that 100 grams of francium-223 will break down so that only 50 grams are left after 22 minutes. Another 22 minutes later, 25 grams of francium-223 will remain, and so on.
Extraction
Francium is not extracted from the Earth's crust.
Uses
Francium has no uses because of its rarity. Scientists hope to learn about the composition of matter by studying the element, however.
Compounds
There are no commercially important compounds of francium.
Health effects
Scientists know too little about francium to be aware of its health effects. As a radioactive element, however, it does pose a threat to human health.