PLUTONIUM

Overview
Plutonium is a synthetic (artificial) element. It exists naturally only in the smallest imaginable amounts. Plutonium was first prepared artificially by a team of researchers at the University of California at Berkeley (UCB) in 1941. News of this discovery was not released, however, until 1946. This delay was caused by the need for secrecy about scientific developments during World War II (1939-45).
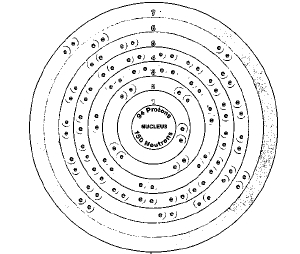
Plutonium is a member of the actinide family. The actinides occur in Row 7 of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. The actinides get their name from element 89, actinium, which is sometimes considered the first member of the family. Plutonium is also called a transuranium element. The term transuranium means "beyond uranium." Elements with atomic numbers greater than that of uranium (92) are called transuranium elements.
SYMBOL
Pu
ATOMIC NUMBER
94
ATOMIC MASS
244.0642
FAMILY
Actinide
Transuranium element
PRONUNCIATION
plu-TOE-nee-um
Plutonium has two important uses. First, some of its isotopes will undergo nuclear fission. Nuclear fission is a process in which an element is bombarded with neutrons. The element breaks apart into simpler elements, releasing large amounts of
Discovery and naming
In 1940, American physicists Edwin McMillan (1907-91) and Philip Abelson (1913- ) discovered the first transuranium element, neptunium (atomic number 93). The neptunium they produced was radioactive. They predicted it would break down to form a new element, atomic number 94. But McMillan and Abelson were called away to do research on the atomic bomb. They suggested to a colleague, Glenn Seaborg (1912- ), that he continue their research on neptunium.
Seaborg and his associates picked up where McMillan and Abelson had left off. They eventually proved that element 94 did exist. The proof came in an experiment they conducted in a particle accelerator at UCB. A particle accelerator is sometimes called an "atom smasher." It is used to cause small particles, such as protons, to move at very high speeds. The particles then collide with targets, such as gold, copper, or tin. When struck by the particles, the targets break apart, forming new elements and other particles.
Seaborg's team suggested the name plutonium for the new element, in honor of the planet Pluto. The two elements just before plutonium in the periodic table had also been named for planets: uranium for Uranus and neptunium for Neptune.
Glenn Seaborg later went on to find a number of other elements. One of those elements, atomic number 106, has been named seaborgium in his honor. (See transfermium elements in this volume.)
Physical properties
Plutonium is a silvery-white metal with a melting point of 639.5°C (1,183°F) and a density of 19.816 grams per cubic centimeter.
Chemical properties
Plutonium is highly reactive and forms a number of different compounds.
Occurrence in nature
Scientists now know that very small amounts of plutonium occur in the Earth's crust. It is formed in ores of uranium. When uranium breaks down, it sometimes forms plutonium in very small quantities. Scientists believe that the abundance of plutonium in the earth is about one quintillionth parts per million.
Isotopes
About 15 isotopes of plutonium are known to exist. All of these isotopes are radioactive. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Plutonium is named after the planet Pluto.
The most stable isotopes of plutonium are plutonium-242 and plutonium-244. The half lives of these two isotopes are 376,300 years and 82,600,000 years respectively. The half life of a radioactive element is the time it takes for half of a sample of the element to break down. Consider the isotope plutonium-242,
Extraction
Plutonium is extracted from natural sources only rarely and only for the purposes of research.
Uses
The most important uses of plutonium depend on two of its properties. First, the radiation given off by plutonium occurs as heat. In fact, plutonium gives off so much heat that the metal feels warm when it is touched. If a large piece of plutonium is placed into water, the heat released can cause the water to boil.
Plutonium provides electrical power on space probes and space vehicles.
This property makes plutonium a good choice for certain thermoelectric generator applications. A thermoelectric generator is a device that converts heat into electricity. Plutonium generators are not practical on a large scale basis. But they are very desirable for special conditions. For example, they have been used to provide electrical power on space probes and space vehicles. They have also been used in artificial pacemakers for people with heart conditions. The isotope most commonly used for this application is plutonium-238 because the radiation it gives off does not pose a threat to people's health.
Plutonium is also used as a fuel in nuclear power plants and in making nuclear weapons ("atomic bombs"). The isotope used for this purpose is plutonium-239. It is used because it will undergo nuclear fission. Very few isotopes will undergo nuclear fission. Two isotopes of uranium, uranium-233 and uranium-235, are among these. But uranium-233 does not occur at all in nature and uranium-235 occurs in only very small amounts.
Using fuel to make fuel
T he production of plutonium fuel (plutonium-239) is a fascinating story. When nuclear reactors were first built, they all used uranium-235 as a fuel. Of the three naturally occurring isotopes of uranium, only uranium-235 will undergo fission.
But the uranium used in a nuclear reactor is never pure uranium. Instead, it is natural uranium with an increased amount of uranium-235. The uranium is said to be "enriched" with uranium-235. But a lot of the main isotope of uranium, uranium-238, remains mixed with the uranium-235.
Fission of uranium-235 occurs when neutrons are fired into the reactor. Neutrons are subatomic particles with no electric charge. They cause uranium-235 to break apart, giving off energy. That energy is then used to make electricity.
But neutrons also collide with uranium-238 isotopes in the reactor. This
isotope does not undergo fission, but does undergo another kind of
change. It soaks up neutrons and changes into plutonium-239:
The plutonium that is formed can be removed from the reactor. It is then purified and re-used as fuel in another nuclear reactor.
What an amazing process this is! One could compare it to the burning of coal to make electricity. In a coal-fired power plant, coal is burned to boil water. Steam runs turbines that make electricity, but when the coal burns up, it's gone.
In a nuclear reactor, the breakdown of uranium-235 atoms gives off energy like the burning of coal. Over time, most of the uranium-235 atoms are used up. But while this is happening, a new fuel is being made! Atoms of plutonium-239 are being produced from atoms of uranium-238. Some reactors are operated primarily to make plutonium, not to make electricity. These reactors are called breeder reactors because they generate new fuel as they operate.
By contrast, plutonium-239 can be made fairly easily in nuclear power reactors. It is a by-product, or "waste product," of these reactors. It can be removed from the reactor, purified, and then re-used to make electrical power.
Compounds
No plutonium compounds have any commercial application.
Plutonium is one of the most toxic elements known. Scientists do not handle it directly. They use remote control devices and stand behind special shielding to protect themselves from the radiation produced by plutonium.
Health effects
Plutonium is one of the most toxic elements known. In the body, it tends to concentrate in bones. One of its most serious health effects on a long-term basis is bone cancer. Scientists who work with plutonium do not handle the metal directly. Instead, they use remote control devices. They always stand behind special shielding to protect themselves from the radiation produced by plutonium.