Lipids

Lipids are a class of biomolecules that is defined by their solubility in organic solvents, such as chloroform, and their relative insolubility in water. Interactions among lipids and of lipids with other biomolecules arise largely from their hydrophobic ("water-hating") nature. Lipids can be divided into two main categories according to their structures: those that are based on fatty acids, and those that are based on isoprene , a branched, five-carbon chain.
Fatty Acid–Based Lipids
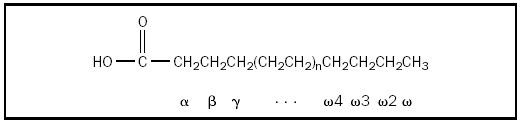
Fatty acids are unbranched carboxylic acids, usually containing an even number of carbon atoms (between 12 and 24, inclusive). If there are no double bonds between carbon atoms, the fatty acid is saturated; if there are double bonds between carbon atoms, the fatty acid is unsaturated. Naturally occurring unsaturated fatty acids have one to six double bonds, with the double bonds separated by at least two single bonds; the double bonds have the cis configuration. These double bonds inhibit "packing" of the molecules (in solids), which lowers the fatty acid melting point . Many physical properties of lipid substances are determined by the extent of unsaturation. Polyunsaturated omega-3 ( ω -3) fatty acids, so named because the double bond between the third to last ( ω -3) and fourth to last ( ω -4) carbons, are commonly found in cold-water fish and are thought to play an important role in many neurological functions.
In response to stress conditions, various tissues convert polyunsaturated fatty acids having twenty carbons to a family of compounds called eicosanoids. Eicosanoids include prostaglandins, thromboxanes, prostacyclins, and leukotrienes, and are generally involved in inflammation and pain sensation. Aspirin, acetaminophen, and other analgesics work by inhibiting the initial reactions required for the conversion of fatty acids to eicosanoids.
The carboxylic acid group of a fatty acid molecule provides a convenient place for linking the fatty acid to an alcohol, via an ester linkage. If the fatty acid becomes attached to an alcohol with a long carbon chain, the resultant substance is called a wax. Waxes are very hydrophobic, and thus repel water. Glycerol, a three-carbon compound with an alcohol group at each carbon, very commonly forms esters with fatty acids. When glycerol and a fatty acid molecule are combined, the fatty acid portion of the resultant compound is called an "acyl" group, and the glycerol portion is referred to as a "glyceride." Using this nomenclature system, a triacylglyceride has three fatty acids attached to a single glycerol molecule; sometimes this name is shortened to "triglyceride." Triglyceride substances are commonly referred to as
fats or oils, depending on whether they are solid or liquid at room temperature. Triglycerides are an energy reserve in biological systems. Diacylglycerides are commonly found in nature with acyl chains occurring at two adjacent carbons, and are the basis of phospholipid chemistry.
Isoprene-Based Lipids
The other class of lipid molecules, based on a branched five-carbon structure called isoprene, was first identified via steam distillation of plant materials. The extracts are called "essential oils." They are often fragrant, and are used as medicines, spices, and perfumes. A wide variety of structures is obtained by fusing isoprene monomer units, leading to a very diverse set of compounds, including terpenes, such as β- carotene, pinene (turpentine), and carvone (oil of spearmint); and steroids, such as testosterone, cholesterol, and estrogen .
Lipid Organization
"Like oil and water" is a saying based on the minimal interaction of lipids with water. Although this saying is apt for isoprene-based lipids and bulky fatty acid–based lipids such as waxes and triglycerides, it is not apt for all lipids (e.g., it does not apply to substances composed of fatty acids or diacylglycerides).
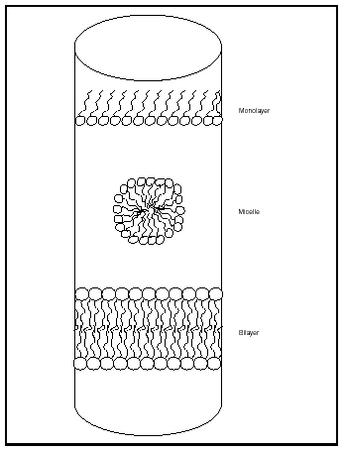
Fatty acids and diacylglycerides are often amphipathic; that is, the carboxylic acid "head" is hydrophilic and the hydrocarbon "tail" is hydrophobic. When a fatty acid or triglyceride substance is placed in water, structures that maximize the interactions of the hydrophilic heads with water and minimize the interactions of the hydrophobic tails with water are formed. At low lipid concentrations a monolayer is formed, with hydrophilic heads associating with water molecules and hydrophobic tails "pointing" straight into the air (see Figure 2).
As the concentration of lipid is increased, the surface area available for monolayer formation is reduced, leading to the formation of alternative structures (depending on the particular lipid and condition). Compounds that have a relatively large head group and small tail group, such as fatty acids and detergents, form spherical structures known as micelles. The concentration of lipid required for micelle formation is referred to as the critical micelle concentration (CMC). Other hydrophobic molecules, such as molecules within dirt, triacylglycerides, and other large organic molecules, associate with the hydrophobic tail portion of a micelle.
Compounds that have approximately equal-sized heads and tails tend to form bilayers instead of micelles. In these structures, two monolayers of lipid molecules associate tail to tail, thus minimizing the contact of the hydrophobic portions with water and maximizing hydrophilic interactions. Lipid molecules can move laterally (within a single layer of the bilayer, called a leaflet), but movement from one leaflet to the opposing leaflet is much more difficult.
H 2 CO: 2(l) + 4 + 6 = 12
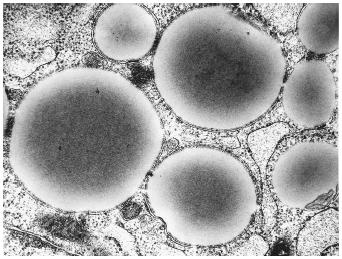
Often these bilayer sheets can wrap around in such a way as to form spherical structures, called vesicles or liposomes (depending on their size). Several new anticancer treatments are based upon the packaging of chemotherapeutic
agents inside liposomes and then directing the liposomes to a specific target tissue.
Lipids can also form structures in conjunction with various proteins. A cell membrane consists of a lipid bilayer that holds within it a variety of proteins that either transverse the bilayer or are associated more loosely with the bilayer. Cholesterol can insert into the bilayer, and this helps to regulate the fluidity of the membrane.
A variety of lipid-protein complexes are used in the body to transport relatively water-insoluble lipids, such as triglycerides and cholesterol, in circulating blood. These complexes are commonly called lipoproteins; they contain both proteins and lipids in varying concentrations. The density of these lipoproteins depends on the relative amounts of protein, because lipids are less dense than protein. Low density lipoproteins, or LDLs, have a relatively higher ratio of lipid to protein. LDLs are used to transport cholesterol and triglycerides from the liver to the tissues. In contrast, high density
lipoproteins, or HDLs, have a relatively lower ratio of lipid to protein and are used in the removal of cholesterol and fats from tissues.
Functions of Lipids
Lipids perform a variety of tasks in biological systems. Terpenes, steroids, and eicosanoids act as communication molecules, either with other organisms or with other cells within the same organism. The highly reduced carbon atoms in triglycerides help to make fats an ideal energy storage compound.
Some of the functions of lipids are related to the structures they form. The micelle formation characteristic of fatty acids, detergents, and soaps in aqueous solution helps to dissolve dirt and other hydrophobic materials. Lipid bilayers play many vital roles. Liposomes are used to deliver drugs to desired tissues. A cell membrane, because of its hydrophobic core, is a substantial barrier to the passage of ions, allowing the cell interior to have concentrations of ions different from those of the extracellular environment. Bilayers are good electrical insulators, and aid in the transmission of nerve impulses along the conducting portions of nerve fibers. The importance of lipids in neural function is seen in diseases in which these insulators are lost, such as multiple sclerosis, or not properly maintained, such as Tay-Sachs disease.
Although they are a chemically diverse assortment of compounds, lipids share a number of properties. The amphipathic nature of lipid molecules encourages the formation of more complex structures such as micelles, bilayers, and liposomes. These structures, as well as the actual lipid substances themselves, affect all aspects of cell biology.
SEE ALSO Fats and Fatty Acids ; Lipid Bilayers ; Membrane ; Phospholipids .
Ann T. S. Taylor
Scott E. Feller
Bibliography
Voet, Donald; Voet, Judith G.; and Pratt, Charlotte (1999). Fundamentals of Biochemistry. New York: Wiley.
Internet Resources
King, Michael W. Lipoproteins. Indiana University School of Medicine. Available from http://www.indstate.edu/thcme/ .
Comment about this article, ask questions, or add new information about this topic: