Phospholipids
Phospholipids are an important class of biomolecules. Phospholipids are the fundamental building blocks of cellular membranes and are the major part of surfactant , the film that occupies the air/liquid interfaces in the lung. These molecules consist of a polar or charged head group and a pair of nonpolar fatty acid tails, connected via a glycerol linkage. This combination of polar and nonpolar segments is termed amphiphilic, and the word describes the tendency of these molecules to assemble at interfaces between polar and nonpolar phases.
The structure of the most common class of phospholipids, phosphoglycerides, is based on glycerol, a three-carbon alcohol with the formula CH 2 OH–CHOH–CH 2 OH. Two fatty acid chains, each typically having an even number of carbon atoms between 14 and 20, attach (via a dual esterification ) to the first and second carbons of the glycerol molecule, denoted as the sn1 and sn2 positions, respectively. The third hydroxyl group of glycerol, at position sn3, reacts with phosphoric acid to form phosphatidate. Common phospholipids, widely distributed in nature, are produced by further reaction of the phosphate group in phosphatidate with an alcohol, such as serine, ethanolamine, choline, glyercol, or inositol. The resulting lipids may be charged, for example, phosphatidyl serine (PS), phosphatidyl inositol (PI), and phosphatidyl glyercol (PG); or dipolar (having separate positively and negatively charged regions), for example, phosphatidyl choline (PC), and phosphatidyl ethanolamine (PE). The term "lecithin" refers to PC-type lipids. A typical phospholipid arrangement is the presence of a saturated fatty acid, such as palmitic or stearic acid, at the sn1 position, and an unsaturated or polyunsaturated fatty acid, such as oleic or arachodonic acid, at sn2 (see Figure 1 for the structure of a phosphoglyceride).
Another class of phospholipids is the sphingolipids. A sphingolipid molecule has the phosphatidyl-based headgroup structure described above, but (in contrast to a common phospholipid molecule) contains a single fatty acid
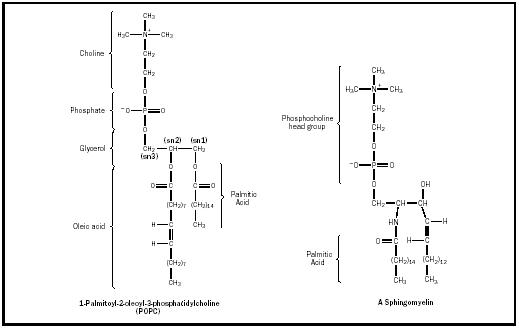
and a long-chain alcohol as its hydrophobic components. Additionally, the backbone of the sphingolipid is sphingosine, an amino alcohol (rather than glyercol). The structure of a representative sphingolipid, sphingomyelin, is also shown in Figure 1. Sphingolipids, occurring primarily in nervous tissue, are thought to form cholesterol-rich domains within lipid bilayer membranes that may be important to the functions of some membrane proteins.
Phospholipids have many functions in biological systems: as fuels, as membrane structural elements, as signaling agents, and as surfactants. For example, pulmonary surfactant is a mixture of lipids (primarily dipalmitoyl phosphatidyl choline [DPPC]) and proteins that controls the surface tension of the fluid lining of the inner lung (the site of gas exchange), allowing rapid expansion and compression of this lining during the breathing cycle. Phospholipids are the major lipid constituent in cell membranes, thus maintaining structural integrity between the cell and its environment and providing boundaries between compartments within the cell.
Bibliography
Berg, Jeremy M.; Tymoczko, John L.; and Stryer, Lubert (2002). Biochemistry, 5th edition. New York: W. H. Freeman.
Voet, Donald; Voet, Judith G.; and Pratt, Charlotte (1999). Fundamentals of Biochemistry. New York: Wiley.
Comment about this article, ask questions, or add new information about this topic: