Lipid Bilayers
Lipid bilayers form the fundamental structures of cell membranes and thus provide a semipermeable interface between the interior and exterior of a cell and between compartments within the cell. Bilayer-forming lipids are amphipathic molecules (containing both hydrophilic and hydrophobic components). The hydrophilic fragment, typically termed the lipid head-group, is charged, or polar, whereas the hydrophobic section consists of a pair of alkyl chains (typically between 14 and 20 carbon atoms in length). A typical class of bilayer-forming lipid is the diacyl phosphatidylcholines, in which phosphate and tetramethyl ammonium moieties comprise the polar head groups and two fatty acids chains constitute the hydrophobic portions.
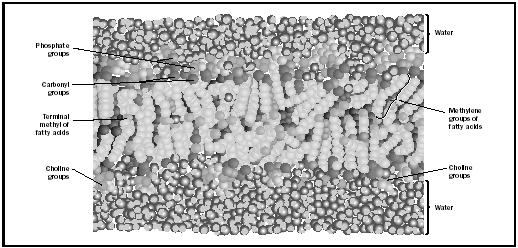
In an aqueous environment the lipids self-assemble into structures that minimize contact between water molecules and the hydrophobic components of the lipids by forming two leaflets (monolayers); this arrangement brings the hydrophobic tails of each leaflet in direct contact with each other, and leaves the head groups in contact with water (see Figure 1). Lipid bilayers are classified as lyotropic and thermotropic liquid crystals, meaning that their structures are a function of both water content and temperature, respectively. Under conditions of low hydration and/or low temperature, the lipid bilayer is ordered with straight alkyl chains and a regular arrangement of head groups, and is in a gel state. At high water content and/or elevated temperatures the bilayer exists in the (biologically relevant) fluid state, characterized by a high degree of disorder in both chain and head group conformations (Figure 1 represents the fluid bilayer state).
The alkyl chain melting point , the gel to fluid transition temperature, is a function of chain length (shorter chains will dissociate at lower temperatures) and the extent of unsaturation within the chains (greater double bond content lowers the melting temperature). The fluid phase bilayer has a hydrophobic thickness on the order of 30 angstroms, and (because of the hydrophobic chains) is a significant barrier to the passage of charged or polar solutes. The bilayer is fluid in the sense that lipid molecules and other membrane constituents readily undergo lateral diffusion within the plane of the membrane.
Bibliography
Berg, Jeremy M.; Tymoczko, John L.; and Stryer, Lubert (2002). Biochemistry, 5th edition. New York: W. H. Freeman.
Voet, Donald; Voet, Judith G.; and Pratt, Charlotte (1999). Fundamentals of Biochemistry. New York: Wiley.
Comment about this article, ask questions, or add new information about this topic: