YTTERBIUM

Overview
Ytterbium belongs to the lanthanide family. The lanthanides make up Row 6 of the periodic table. The periodic table is a chart that shows how the chemical elements are related to each other. The lanthanides are also known as the rare earth elements. The name suggests that the lanthanides do not occur commonly in the earth. In fact, that is not correct. They are not all that uncommon. The name rare earth arose because the elements are so difficult to separate from each other. With modern techniques, this separation can be done much more easily.
Ytterbium was one of nine new elements discovered in the mineral yttria at the end of the nineteenth century. Analyzing this mineral posed great difficulties for chemists of the time. The elements in yttria have very similar properties. That makes it difficult to separate them from each other. Three chemists, Jean-Charles Gallisard de Marignac, Lars Fredrik Nilson, and Georges Urbain, all deserve partial credit for discovering ytterbium.
SYMBOL
Yb
ATOMIC NUMBER
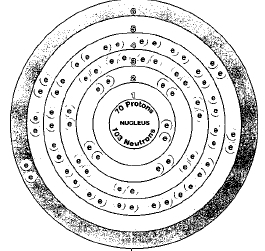
70
ATOMIC MASS
173.04
FAMILY
Lanthanide
(rare earth metal)
PRONUNCIATION
i-TER-bee-um
Discovery and naming
In 1878, French chemist Jean-Charles-Galissard de Marignac (1817-94) reported his analysis of the mineral erbia. Erbia was one of the minerals found a century earlier in an interesting new rock called yttria. The rock had been discovered outside the town of Ytterby, Sweden, by Swedish army officer Carl Axel Arrhenius (1757-1824) in 1787. In the century that followed Arrhenius' discovery, chemists worked hard to find out what elements were in yttria. Earlier chemists thought erbia was a new element, but Marignac disagreed. He said that erbia consisted of two new elements, which he called erbium and ytterbium.
The very next year, a second Swedish chemist, Lars Fredrik Nilson (1840-99), proved that Marignac was wrong. Ytterbium was not a new element, he said. Instead, it consisted of two other new elements. Nilson called these elements scandium and ytterbium. (See sidebar on Nilson in the scandium entry.)
Nilson's analysis still did not solve this confusion. In 1907, French chemist Georges Urbain (1872-1938) announced that Nilson's ytterbium was also a mixture of two new elements. Urbain called these elements ytterbium and lutetium. Marignac, Nilson, and Urbain are all given part of the credit for the discovery of ytterbium.
In fact, the ytterbium studied by Marignac, Nilson, and Urbain was not pure ytterbium. Instead, it was combined with oxygen and other elements. Fairly pure ytterbium metal was not produced until 1937 and high purity ytterbium was not produced until 1953.
Physical properties
Ytterbium is a typical metal. It has a bright, shiny surface and is malleable and ductile. Malleable means capable of being hammered into thin sheets. Ductile means capable of being drawn into thin wires. Its melting point is 824°C (1,515°F) and its boiling point is 1,427°C (2,600°F). It has a density of 7.01 grams per cubic centimeter.
Chemical properties
Ytterbium tends to be more reactive than other lanthanide elements. It is usually stored in sealed containers to keep it from reacting with oxygen in the air. It also reacts slowly with water and more rapidly with acids and liquid ammonia.
Occurrence in nature
Ytterbium is one of the more common lanthanides. It is thought to have an abundance of about 2.7 to 8 parts per million in the Earth's crust. That makes it somewhat more common than bromine, uranium, tin, and arsenic. Its most common ore is monazite, which is found in beach sands in Brazil, India, and Florida. Monazite typically contains about 0.03 percent ytterbium.
Isotopes
Seven naturally occurring isotopes of ytterbium are known. These isotopes are ytterbium-168, ytterbium-170, ytterbium-171, ytterbium-172, ytterbium-173, ytterbium-174, and ytterbium-176. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Ten radioactive isotopes of ytterbium are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
Studies have been done on one radioactive isotope of ytterbium, ytterbium-169, for possible use in a portable X-ray machine. This isotope gives off gamma radiation, which is similar to X rays. Gamma rays pass through soft tissues in the body, just like X rays. But they are blocked by bones and other thick material. A small amount of ytterbium-169 acts just like a tiny X-ray machine. It can be carried around more easily than can a big X-ray machine.
Extraction
Ytterbium is obtained from its ores by reaction with
lanthanum
metal:
Ytterbium's most common ore is found in beach sands in Brazil, India, and Florida.
Uses
Ytterbium has no major commercial uses. A small amount is used to add strength to special types of steel. Some ytterbium is also used in making lasers. A laser is a device for producing very bright light of a single color. The kind of light produced by a laser depends on the elements used in making it. A laser made with ytterbium has properties different from those without ytterbium.
Compounds
The only ytterbium compound of commercial interest is ytterbium oxide (Yb 2 O 3 ). This compound is used to make alloys and special types of ceramics and glass.
Health effects
Ytterbium is not thought to be a very toxic element.