SCANDIUM

Overview
The existence of scandium was predicted nearly ten years before it was actually discovered. The prediction was made by Russian chemist Dmitri Mendeleev (1834-1907). Mendeleev developed the periodic table based on his periodic law. The periodic table is a chart that shows how chemical elements are related to one another. The table originally had a number of empty boxes for elements that had not been discovered. Chemists were able to search for these elements based on the properties of the elements around the empty boxes. Scandium was found in 1879 by Swedish chemist Lars Nilson (1840-99). It is a transition metal, appearing in Group 3 (IIIB).
Scandium is a moderately abundant element. However, it tends to be spread out throughout the earth rather than concentrated in a few places. This makes it difficult to isolate. In fact, scandium is classified as a rare earth element. Rare earth elements are not really "rare." However, they are difficult to extract from the earth. They are also difficult to separate from each other.
SYMBOL
Sc
ATOMIC NUMBER
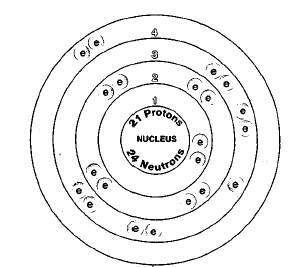
21
ATOMIC MASS
44.9559
FAMILY
Group 3 (IIIB)
Transition metal
PRONUNCIATION
SCAN-dee-um
Scandium has few commercial uses. It is sometimes combined with other metals to make alloys. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals. Scandium alloys are being used more in various kinds of sporting equipment and in other applications.
Discovery and naming
In 1869, Mendeleev made one of the great discoveries in the history of chemistry, the periodic law. The periodic law shows how the chemical elements are related to each other. The most common way of representing the periodic law is in a chart called the periodic table.
Mendeleev's original periodic table contained only about 60 elements. That was the total number of elements known in 1869. When he drew his first periodic table, Mendeleev found some empty places. What did those empty places mean?
Mendeleev made a prediction that the empty places in the periodic table stood for elements that had not yet been discovered. He said one could tell what those elements are going to be like by examining their position in the periodic table. For example, element number 21 would be like boron , Mendeleev predicted. Boron was the element above number 21 in Mendeleev's chart. He called the missing element (number 21) ekaboron, or "similar to boron."
Chemists were fascinated by Mendeleev's prediction. Could he really tell them how to look for a new element? And could he tell them what that element would be like?
One of the chemists who took up the challenge was Nilson. Nilson analyzed two minerals known as gadolinite and euxenite, in search of the missing element. By 1879, he announced the discovery of "ekaboron." He suggested the name scandium, in honor of Scandinavia, the region in which Nilson' homeland of Sweden is located. (See accompanying sidebar on Nilson.)
Nilson's discovery was very important in chemistry. It showed that Mendeleev's periodic law was correct. The law did show how elements are related to each other. It could be used to describe elements that had not even been discovered!
The substance discovered by Nilson was not pure scandium metal, but a compound of scandium and oxygen —scandium oxide (Sc 2 O 3 ). It is quite difficult to produce pure scandium metal from scandium oxide. In fact, it was not until 1937 that the metal was isolated. Then, it was another twenty years before a large sample (weighing one pound) was produced. Today, companies that use scandium often buy the oxide rather than the pure metal. The oxide costs several thousand dollars per kilogram. By comparison, the pure metal costs a few hundred thousand dollars per kilogram.
Physical properties
Scandium metal is a silvery-white solid with a slight pink or yellow tint when exposed to air. It has a melting point of 1,538°C (2,800°F) and a boiling point of about 2,700°C (4,900°F). Its density is 2.99 grams per cubic centimeter.
Lars Nilson | Swedish chemist
L ars Nilson was born in the Swedish town of Östergötland on May 27, 1840. He entered the University of Upsala at the age of 19, intending to study biology, chemistry, and geology. He found university work difficult because he was in very poor health. He often suffered from bleeding in the lungs.
Yet, he persevered and was ready to receive his doctoral degree in 1865. Then he received word that his father was seriously ill. Instead of finishing his university work, he returned home. He took charge of the farm and helped his sick father for many months. At the end of that time, he made a surprising discovery. His illness had disappeared. He was healthy enough to return to Upsala and earn his degree.
In 1879, Nilson made the discovery for which he is most famous. He was studying a mineral known as erbia. The mineral was a complex mixture of many elements. Many chemists throughout Europe were trying to find out exactly what elements were present in erbia.
Nilson found a new element in erbia that no one had yet seen. He was surprised to discover that the element had already been predicted. Russian chemist Dmitri Mendeleev had discovered the periodic law only ten years earlier. Mendeleev had used the periodic law to predict the existence of three elements that had not yet been discovered. One of these elements exactly matched the element found by Nilson. Nilson named the element scandium in honor of his native region, Scandinavia.
Chemical properties
Scandium is similar to the rare earth elements chemically. It reacts readily with acids, but does not react easily with oxygen in the air.
Occurrence in nature
The abundance of scandium is thought to be about 5 to 6 parts per million in the Earth's crust. Interestingly, the element seems to be much more abundant in the sun and some stars than it is on Earth.
Scandium is thought to occur in more than 800 different minerals. Its most important ores are the minerals thortveitite and wolframite. It is also found in minerals containing other rare earth elements, such as monazite, bastnasite, and gadolinite.
In the United States, scandium is obtained from the waste products of other mining operations. Some scandium comes from the mining of fluorite at Crystal Mountain, Montana, and some from the mining of tantalum in Muskogee, Oklahoma. The actual amount of scandium produced in the United States is not announced. It is regarded as a trade secret in the industry.
Isotopes
Only one naturally occurring isotope of scandium is known, scandium-45. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
About 10 radioactive isotopes of scandium are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
There are no commercial uses for any radioactive isotope of scandium.
Scandium is thought to occur in more than 800 different minerals.
Extraction
Pure scandium metal can be made by reacting scandium fluoride (ScF
3
) with another active metal, such as
calcium
or
zinc:
Uses
There are relatively few commercial uses for scandium or its compounds. It is sometimes used to make alloys for special purposes. Scandium metal is lighter than most other metals. It is also resistant to corrosion (rusting) and has a high melting point. These properties make scandium alloys especially desirable for use in sporting equipment, such as baseball bats, lacrosse sticks, and bicycle frames. These alloys may also have some applications in the aerospace industry. These applications are not yet well developed, however, because of the high cost of the metal.
Scandium alloys are also used in specialized lamps. The presence of scandium produces light that is very similar to that of natural sunlight.
Compounds
None of the compounds of scandium has any important commercial use.
Health effects
As with the rare earth elements, little is known about the health effects of scandium. In such cases, the best policy is to handle the metal very carefully.