TIN
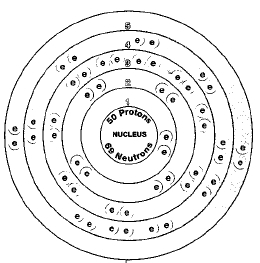
Overview
Tin is a member of Group 14 (IVA) in the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. Tin is also part of the the carbon family. Other carbon family elements include carbon, silicon, germanium, and lead.
Tin is a highly workable metal that was once as valuable as silver for jewelry, coins, and special dishware. Today it is used as sheets in the construction of buildings and roofs, for soldering or joining metal parts, for storage containers, and in alloys like bronze and Babbitt metal.
Discovery and naming
Tin, its alloys, and its compounds have been known to humans for thousands of years. A number of references to the element can be found in the Bible. Tin was apparently known to other civilizations also. For example, the sacred Hindu book Rig Veda, written in about 1000 B.C., mentions tin among other metals known to the Hindus.
SYMBOL
Sn
ATOMIC NUMBER
50
ATOMIC MASS
118.69
FAMILY
Group 14 (IVA)
Carbon
PRONUNCIATION
TIN
The alloy of tin known as bronze was probably produced even earlier than the pure metal. An alloy is made by melting and mixing two or more metals. The mixture has properties that are different than any of the metals alone. The Egyptians, Mesopotamians, Babylonians, and Peruvians were producing bronze as far back as 2000 B.C. The alloy was probably discovered accidentally when copper and tin compounds were heated together. Over time, a method for producing consistent bronze was developed.
Bronze became popular among ancient peoples because it was harder and tougher than copper. Before the discovery of bronze, many metal items were made out of copper. But copper is soft and bends easily. Bronze is a much better replacement for copper in tools, eating utensils, and weapons. Bronze marked a significant advance in human civilization. This strong alloy improved transportation methods, food preparation, and quality of life during a period now known as the Bronze Age (4000—3000 B.C. ).
The origin of the name tin is lost in history. Some scholars believe it is named for the Etruscan god Tinia. During the Middle Ages, the metal was known by its Latin name, stannum. It is from this name that the element's symbol, Sn, is derived.
Physical properties
The most common allotrope of tin is a silver-white metallic-looking solid known as the β-form (or "beta-form"). Allotropes are forms of an element with different physical and chemical properties. This "white tin" has a melting point of 232°C (450°F), a boiling point of 2,260°C (4,100°F), and a density of 7.31 grams per cubic centimeter.
One of tin's most interesting properties is its tendency to give off a strange screeching sound when it is bent. This sound is sometimes known as "tin cry." β-tin is both malleable and ductile. Malleable means capable of being hammered into thin sheets. Ductile means capable of being drawn into a thin wire. At temperatures greater than 200°C, tin becomes very brittle.
A second form of tin is α-tin (or "alpha-tin"), also known as "gray tin." Gray tin forms when white tin is cooled to temperatures less than about 13°C. Gray tin is a gray amorphous (lacking a crystalline shape) powder. The change from white tin to gray tin takes place rather slowly. This change is responsible for some peculiar and amazing changes in objects made from
In the late nineteenth century, organ pipes in many cathedrals of Northern Europe were made of tin alloys. During the coldest winters, these pipes began to crumble as tin changed from one allotropic form to the other. The change was known as "tin disease." At the time, no one knew why this change occurred.
One of tin's most interesting properties is its tendency to give off a strange screeching sound when it is bent. This sound is sometimes known as "tin cry."
Chemical properties
Tin is relatively unaffected by both water and oxygen at room temperatures. It does not rust, corrode, or react in any other way. This explains one of its major uses: as a coating to protect other metals. At higher temperatures, however, the metal reacts with both water (as steam) and oxygen to form tin oxide.
Similarly, tin is attacked only slowly by dilute acids such as hydrochloric acid (HCl) and sulfuric acid (H 2 SO 4 ). Dilute acids are mixtures that contain small amounts of acid dissolved in large amounts of water. This property also makes tin a good protective covering. It does not react with acids as rapidly as do many other kinds of metals, such as iron, and can be used, therefore, as a covering for those metals.
Tin dissolves easily in concentrated acids, however, and in hot alkaline solutions, such as hot, concentrated potassium hydroxide (KOH). The metal also reacts with the halogens to form compounds such as tin chloride and tin bromide. It also forms compounds with sulfur, selenium, and tellurium.
Occurrence in nature
Tin is not very abundant in nature. It ranks about 50th on the list of elements most commonly found in the Earth's crust. Estimates are that the crust contains about 1 to 2 parts per million of tin.
By far the most common ore of tin is cassiterite, a form of tin oxide (SnO 2 ). An ore is a compound or mixture from which an element can be extracted for commercial profit. Cassiterite has been mined for thousands of years as a source of tin. During ancient times, Europe obtained most of its tin from the British Isles. Today, the major producers of tin are China, Indonesia, Peru, Brazil, and Bolivia. The United States produces almost no tin of its own although it is the major consumer of the metal.
Isotopes
Tin has ten naturally occurring isotopes. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Fifteen radioactive isotopes have also been discovered. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
None of the radioactive isotopes of tin have any commercial applications.
Extraction
Tin can be produced easily by heating cassiterite with charcoal (nearly
pure
carbon).
In this reaction, the carbon reacts with and removes
oxygen
from the cassiterite, leaving pure tin behind.
This reaction occurs so easily that people knew of the reaction thousands of years ago.
In order to obtain very pure tin, however, one problem must be solved.
Iron
often occurs in very small amounts along with tin oxide in cassiterite.
Unless the iron is removed during the extraction process, a very hard,
virtually unusable form of tin is produced. Modern systems of tin
production, therefore, involve two steps. In one of those steps, impure
tin is heated in the presence of oxygen to oxidize any iron in the
mixture. In this reaction, iron is converted to iron(III) oxide, and
metallic tin is left behind:
Uses
The largest amount of tin used in the United States goes to the production of solder. Solder is an alloy, usually made of tin and lead, with a low melting point. It is used to join two metals to each other. For example, metal wires are attached to electrical devices by means of solder. Solder is also used by plumbers to seal the joint between two metal pipes.
The largest amount of tin used in the United States goes to the production of solder.
Solder is often applied by means of a soldering iron. A soldering iron consists of a steel bar through which an electric current runs. The electric current heats the bar as it passes through it. When a small piece of solder is placed on the tip of the soldering iron, it melts. The solder is then applied to the joint between two metals. When it cools, the bond is strong. In 1996, 15,600 metric tons of tin were used in the production of solder.
Tin is also used in the manufacture of other alloys. Bronze, for example, is an alloy of tin and copper. In 1996, more than 2,750 metric tons of bronze were produced in the United States. It is used in a wide variety of industrial products, such as spark-resistant tools, springs, wire, electrical devices, water gauges, and valves.
One application of tin that was once important is in the manufacture of "tin foil." Tin foil is a very thin sheet of tin used to wrap candies, tobacco, and other products. The tin protected the products from spoiling by exposure to air. Today, most tin foil is actually thin sheets of aluminum because aluminum is less expensive.
A very important application of tin is tinplating. Tinplating is the process by which a thin coat of tin is placed on the surface of steel, iron, or another metal. Tin is not affected by air, oxygen, water, acids, and bases to the extent that steel, iron, and other metals are. So the tin coating acts as a protective layer.
Perhaps the best known example of tin plating is in the production of food cans. Tin cans are made of steel and are covered with a thin layer of tin. Most food and drink cans today are made out of aluminum because it is cheaper.
Metals can be plated with tin in one of two ways. First, the metal to be plated can simply be dipped in molten (liquid) tin and then pulled out. A thin layer of liquid tin sticks to the base metal and then cools to form a thin coating. The second method is electroplating. In the process of electroplating, the base metal is suspended in a solution of tin sulfate, or a similar compound. An electric current passes through the solution, causing the tin in the solution to be deposited on the surface of the base metal.
Tin was once important in the manufacture of "tin foil." Now, aluminum is used because it is less expensive.
Another tin alloy is Babbitt metal. Babbitt metal is a soft alloy made of any number of metals, including arsenic, cadmium, lead, or tin. Babbitt metal is used to make ball bearings for large industrial machinery. The Babbitt metal is laid down as a thin coating on heavier metal, such as iron or steel. The Babbitt metal retains a thin layer of lubricating oil more efficiently than iron or steel.
Compounds
About a sixth of all tin consumed in the United States is used in the production of tin compounds. Some of the most important of those compounds and their uses are as follows:
Tin toys
M ost of today's toys are made of plastic. But until World War II (1939-45), most of the finest toys in the world were made of tin-plated metal. The earliest of these toys were made in the early 1800s. They were based on common objects and events, such as trains, horse-drawn carriages, sailing ships, and people from everyday life.
During the first half of the twentieth century, the most popular tin toy was the automobile. Toymakers made replicas of every type of car manufactured in the world. These toy cars ranged from the very simplest to the most detailed and elabrate.
Following World War II, plastic toys became much more popular, but tin toys were still made. Reflecting the times, these toys often represented space ships, robots, and other modern objects.
The manufacture of tin toys is no longer the large-scale business it was one hundred years ago. However, toy-collecting has remained a fascinating hobby for adults and children around the world. Some antique collectors and dealers specialize in tin toys.
tin chloride (SnCl 2 ): used in the manufacture of dyes, polymers, and textiles; in the silvering of mirrors; as a food preservative; as an additive in perfumes used in soaps; and as an anti-gumming agent in lubricating oils
tin oxide (SnO 2 ): used in the manufacture of special kinds of glass, ceramic glazes and colors, perfumes and cosmetics, and textiles; and as a polishing material for steel, glass, and other materials
tin chromate (SnCrO 4 or Sn(CrO 4 ) 2 ): brown or yellowish-brown compounds used as a coloring agent for porcelain and china
tin fluoride (SnF 2 ) and tin pyrophosphate (Sne 2 P 2 O 7 ): used as toothpaste additives to help protect against cavities
Health effects
Most compounds of tin are toxic (poisonous). Tin compounds are most likely to present a hazard when they get into the air. Then, they may be inhaled, after which they can cause problems such as nausea, diarrhea, vomiting, and cramps.
The U.S. government has set a standard of 2 milligrams per cubic meter of air for most tin compounds. For organic compounds of tin (those that contain the element carbon also), the Limit is 0.1 milligram per cubic meter. Miners and factory workers are the most likely people to be exposed to these levels of tin. The amount of tin absorbed from canned foods is too small to be of concern to consumers.
I'm am used this site any conclusion or any problem any where.