ALUMINUM

Overview
Aluminum is found in Row 2, Group 13 of the periodic table. The periodic table is a chart that shows how the chemical elements are related to each other. Elements in the same column usually have similar chemical properties. The first element in this group is boron. However, boron is very different from all other members of the family. Therefore, group 13 is known as the aluminum family.
Aluminum is the third most abundant element in the Earth's crust, falling behind oxygen and silicon. It is the most abundant metal. It is somewhat surprising, then, that aluminum was not discovered until relatively late in human history. Aluminum occurs naturally only in compounds, never as a pure metal. Removing aluminum from its compounds is quite difficult. An inexpensive method for producing pure aluminum was not developed until 1886.
SYMBOL
Al
ATOMIC NUMBER
13
ATOMIC MASS
26.98154
FAMILY
Group 13 (IIIA)
Aluminum
PRONUNCIATION
uh-LOO-min-um
Today, aluminum is the most widely used metal in the world after iron. It is used in the manufacture of automobiles, packaging materials, electrical equipment, machinery, and building construction. Aluminum is also ideal for beer and soft drink cans and foil because it can be melted and reused, or recycled.
Discovery and naming
Aluminum was named for one its most important compounds, alum. Alum is a compound of potassium, aluminum, sulfur, and oxygen. The chemical name is potassium aluminum sulfate, KAl(SO 4 ) 2 .
No one is sure when alum was first used by humans. The ancient Greeks and Romans were familiar with the compound alum. It was mined in early Greece where it was sold to the Turks. The Turks used the compound to make a beautiful dye known as Turkey red. Records indicate that the Romans were using alum as early as the first century B.C.
These early people used alum as an astringent and as a mordant. An astringent is a chemical that causes skin to pull together. Sprinkling alum over a cut causes the skin to close over the cut and start its healing. A mordant is used in dyeing cloth. Few natural dyes stick directly to cloth. A mordant bonds to the cloth and the dye bonds to the mordant.
Over time, chemists gradually began to realize that alum might contain a new element. In the mid-1700s, German chemist Andreas Sigismund Marggraf (1709-82) claimed to have found a new "earth" called alumina in alum. But he was unable to remove a pure metal from alum.
The first person to accomplish that task was Danish chemist and physicist Hans Christian Oersted (1777-1851). Oersted heated a combination of alumina and potassium amalgam. An amalgam is an alloy of a metal and mercury. In this reaction, Oersted produced an aluminum amalgam—aluminum metal in combination with mercury. He was unable, however, to separate the aluminum from the mercury.
Today, aluminum is the most widely used metal in the world after iron.
Pure aluminum metal was finally produced in 1827 by German chemist
Friedrich Wöhler (1800-82). Wöhler used a method perfected by
English chemist Sir Humphry Davy (1778-1829), who succeeded in isolating
several elements during his life-time. (See sidebar on Davy in the
calcium
entry.) Wöhler heated a mixture of aluminum chloride and potassium
metal. Being more active, the potassium replaces the aluminum, as shown by
the following:
The pure aluminum can then be collected as a gray powder, which must be melted to produce the shiny aluminum that is most familiar to consumers.
After Wöhler's work, it was possible, but very expensive, to produce pure aluminum. It cost so much that there were almost no commercial uses for it.
A number of chemists realized how important it was to find a less expensive way to prepare aluminum. In 1883, Russian chemist V. A. Tyurin found a less expensive way to produce pure aluminum. He passed an electric current through a molten (melted) mixture of cryolite and sodium chloride (ordinary table salt). Cryolite is sodium aluminum fluoride (Na 3 AlF 6 ). Over the next few years, similar methods for isolating aluminum were developed by other chemists in Europe.
The most dramatic breakthrough in aluminum research was made by a college student in the United States. Charles Martin Hall (1863-1914) was a student at Oberlin College in Oberlin, Ohio, when he became interested in the problem of producing aluminum. Using homemade equipment in a woodshed behind his home, he achieved success by passing an electric current through a molten mixture of cryolite and aluminum oxide (Al 2 O 3 ).
Hall's method was far cheaper than any previous method. After his discovery, the price of aluminum fell from about $20/kg ($10/lb) to less than $1/kg (about $.40/lb). Hall's research changed aluminum from a semi-precious metal to one that could be used for many everyday products.
What's in a name?
In North America, aluminum is spelled with one i and is pronounced uh-LOO-min-um. Elsewhere in the world, a second i is added—making it aluminium—and the word is pronounced al-yoo-MIN-ee-um.
Physical properties
Aluminum is a silver-like metal with a slightly bluish tint. It has a melting point of 660°C (1,220°F) and a boiling point of 2,327-2,450°C (4,221-4,442°F). The density is 2.708 grams per cubic centimeter. Aluminum is both ductile and malleable. Ductile means capable of being pulled into thin wires. Malleable means capable of being hammered into thin sheets.
Aluminum is an excellent conductor of electricity. Silver and copper are better conductors than aluminum but are much more expensive. Engineers are looking for ways to use aluminum more often in electrical equipment because of its lower costs.
Chemical properties
Aluminum has one interesting and very useful property. In moist air, it
combines slowly with oxygen to form aluminum oxide:
The aluminum oxide forms a very thin, whitish coating on the aluminum metal. The coating prevents the metal from reacting further with oxygen and protects the metal from further corrosion (rusting). It is easy to see the aluminum oxide on aluminum outdoor furniture and unpainted house siding.
Aluminum is a fairly active metal. It reacts with many hot acids. It also reacts with alkalis. An alkali is a chemical with properties opposite those of an acid. Sodium hydroxide (common lye) and limewater are examples of alkalis. It is unusual for an element to react with both acids and alkalis. Such elements are said to be amphoteric.
Aluminum also reacts quickly with hot water. In powdered form, it catches fire quickly when exposed to a flame.
Aluminum: Precious metal?
B efore chemists developed inexpensive ways to produce pure aluminum, it was considered a somewhat precious metal. In fact, in 1855, a bar of pure aluminum metal was displayed at the Paris Exposition. It was placed next to the French crown jewels!
Aluminum is an excellent conductor of electricity.
Occurrence in nature
The abundance of aluminum in the Earth's crust is estimated to be about 8.8 percent. It occurs in many different minerals.
Bauxite, a complicated mixture of compounds consisting of aluminum, oxygen, and other elements, is the primary commercial source for aluminum.
Large reserves of bauxite are found in Australia, Brazil, Guinea, Jamaica, Russia, and the United States. The largest producer of aluminum metal is the United States; states that produce the most aluminum are Montana, Oregon, Washington, Kentucky, North Carolina, South Carolina, and Tennessee.
Isotopes
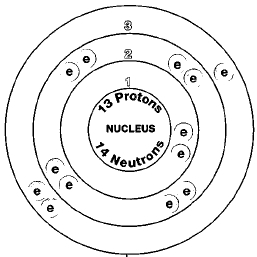
Only one naturally occurring isotope of aluminum exists, aluminum-27. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Aluminum has six radioactive isotopes. A radioactive isotope gives off either energy or subatomic particles in order to reduce the atomic mass and become stable. When the emission produces a change in the number of protons, the atom is no longer the same element. The particles and energy emitted from the nucleus are called radiation. The process of decaying from one element into another is known as radioactive decay.
No radioactive isotope of aluminum has any commercial use.
Extraction
Aluminum production is a two-step process. First, aluminum oxide is separated from bauxite by the Bayer process. In this process, bauxite is mixed with sodium hydroxide (NaOH), which dissolves the aluminum oxide. The other compounds in bauxite are left behind.
The aluminum oxide is then treated with a process similar to the Hall method. There is not enough natural cryolite to make all the aluminum needed, so synthetic (artificial) cryolite is manufactured for this purpose. The chemical reaction is the same with synthetic cryolite as with natural cryolite. About 21
Uses
Aluminum is used as pure metal, in alloys, and in a variety of compounds. An alloy is made by melting and then mixing two or more metals. The mixture has properties different from those of the individual metals. Aluminum alloys are classified in numbered series according to the other elements they contain.
The 1000 classification is reserved for alloys of nearly pure aluminum metal. They tend to be less strong than other alloys of aluminum, however. These metals are used in the structural parts of buildings, as decorative trim, in chemical equipment, and as heat reflectors.
The 2000 series are alloys of copper and aluminum. They are very strong, are corrosion (rust) resistant, and can be
The 3000 series is made up of alloys of aluminum and manganese. These alloys are not as strong as the 2000 series, but they also have good machinability. Alloys in this series are used for cooking utensils, storage tanks, aluminum furniture, highway signs, and roofing.
Alloys in the 4000 series contain silicon. They have low melting points and are used to make solders and to add gray coloring to metal. Solders are low-melting alloys used to join two metals to each other. The 5000, 6000, and 7000 series include alloys consisting of magnesium, both magnesium and silicon, and zinc, respectively. These are used in ship and boat production, parts for cranes and gun mounts, bridges, structural parts in buildings, automobile parts, and aircraft components.
The largest single use of aluminum is in the transportation industry (28 percent). Car and truck manufacturers like aluminum
Twenty-three percent of all aluminum produced finds its way into packaging. Aluminum foil, beer and soft drink cans, paint tubes, and containers for home products such as aerosol sprays are all made from aluminum.
Fourteen percent of all aluminum goes into building and construction. Windows and door frames, screens, roofing, and siding, as well as the construction of mobile homes and structural parts of buildings rely on aluminum.
The remaining 35 percent of aluminum goes into a staggering range of products, including electrical wires and appliances, automobile engines, heating and cooling systems, bridges, vacuum cleaners, kitchen utensils, garden furniture, heavy machinery, and specialized chemical equipment.
Compounds
A relatively small amount of aluminum is used to make a large variety of aluminum compounds. These include:
aluminum ammonium sulfate (Al(NH 4 )(SO 4 ) 2 ): mordant, water purification and sewage treatment, paper production, food additive, leather tanning
aluminum borate (Al 2 O 3 B 2 O 3 ): production of glass and ceramics
aluminum borohydride (Al(BH 4 ) 3 : additive in jet fuels
aluminum chloride (AlCl 3 ): paint manufacture, antiperspirant, petroleum refining, production of synthetic rubber
aluminum fluorosilicate (Al 2 (SiF 6 ) 3 ): production of synthetic gemstones, glass, and ceramics
aluminum hydroxide (Al(OH) 3 ): antacid, mordant, water purification, manufacture of glass and ceramics, waterproofing of fabrics
aluminum phosphate (AlPO 4 ): manufacture of glass, ceramics, pulp and paper products, cosmetics, paints and varnishes, and in making dental cement
aluminum sulfate, or alum (Al 2 (SO 4 ) 3 ): manufacture of paper, mordant, fire extinguisher system, water purification and sewage treatment, food additive, fireproofing and fire retardant, and leather tanning
Health effects
Aluminum has no known function in the human body. There is some debate, however, as to its possible health effects. In the 1980s, some health scientists became concerned that aluminum might be associated with Alzheimer's disease. This is a condition that most commonly affects older people, leading to forgetfulness and loss of mental skills. It is still not clear whether aluminum plays any part in Alzheimer's disease.
Some authorities believe that breathing aluminum dust may also cause health problems. It may cause a pneumonia-like condition currently called aluminosis. Again, there is not enough evidence to support this view.
i am noone