TITANIUM
Overview
Titanium is found in the middle of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. Titanium is a transition metal and is part of Group 4 (IVB).
Titanium was one of the first elements to be discovered by modern chemists. The "modern" chemistry period begins after the middle of the eighteenth century. That period is chosen because it is the first time that the basic concepts of modern chemistry were developed.
Titanium was discovered by English clergyman William Gregor (1761-1817). Gregor studied minerals as a hobby. He did not think of himself as a chemist, and yet his research led to the discovery of titanium.
SYMBOL
Ti
ATOMIC NUMBER
22
ATOMIC MASS
47.88
FAMILY
Group 4 (IVB)
Transition metal
PRONUNCIATION
ty-TAY-nee-um
Titanium and its compounds have become very important in modern society. The metal is widely used in a variety of alloys. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals. Titanium alloys are used in aircraft, spacecraft, jewelry, clocks, armored vehicles, and in the construction of buildings.
Discovery and naming
Gregor discovered titanium while he was studying a mineral found near his home. He was able to identify most of the mineral, but he found one part that he could not identify. He decided it was a new substance, but did not continue his research. Instead, he wrote a report and left it to professional chemists to find out more about the material.
Today, we know that the material Gregor found is a mineral called ilmenite. Ilmenite is made of iron, oxygen, and titanium. Its chemical formula is FeTiO 3 . Even though Gregor did not complete his study of ilmenite, he is usually given credit for the discovery of titanium.
Surprisingly, most chemists paid little attention to Gregor's report. Four years later, German chemist Martin Heinrich Klaproth (1743-1817) decided to study ilmenite. Klaproth believed that Gregor had been correct and that ilmenite truly did contain a new element. Klaproth suggested the name titanium, in honor of the Titans. The Titans were mythical giants who ruled the Earth until they were overthrown by the Greek gods. Klaproth reminded everyone that Gregor should receive credit for having discovered the element.
Klaproth was never able to produce pure titanium from ilmenite, only titanium dioxide (TiO 2 ). It was not until 1825 that even impure titanium metal was produced. Swedish chemist Jöns Jakob Berzelius (1779-1848) accomplished this task.
Physical properties
Pure titanium metal can exist as a dark gray, shiny metal or as a dark gray powder. It has a melting point of 1,677°C (3,051°F) and a boiling point of 3,277°C (5,931°F). Its density is 4.6 grams per cubic centimeter. Titanium metal is brittle when cold and can break apart easily at room temperature. At higher temperatures, it becomes malleable and ductile. Malleable means capable of being hammered into thin sheets. Ductile means capable of being drawn into thin wires.
Titanium has an interesting physical property. Small amounts of oxygen or nitrogen, make it much stronger.

Chemical properties
In general, titanium tends to be quite unreactive. It does not combine with oxygen at room temperature. It also resists attack by acids, chlorine, and other corrosive agents. A corrosive agent is a material that tends to vigorously react or eat away at something.
Titanium becomes more reactive at high temperatures. It can actually catch fire when heated in the presence of oxygen.
Occurrence in nature
Titanium is a very common element. It is the ninth most abundant element in the Earth's crust. Its abundance is estimated to be about 0.63 percent. That places titanium just above hydrogen and just below potassium among elements present in the earth.
Titanium metal is brittle when cold and can break apart easily at room temperature.
The most common mineral sources of titanium are ilmenite, rutile, and titanite. Titanium is also obtained from iron ore slags. Slag is an earthy material that floats to the top when iron is removed from iron ore.
Isotopes
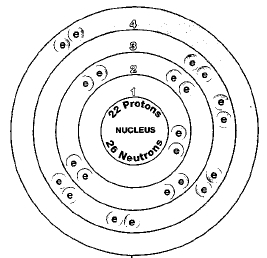
Five naturally occurring isotopes of titanium exist. They are titanium-46, titanium-47, titanium-48, titanium-49, and titanium-50. The most abundant of these is titanium-48. It makes up about 75 percent of all titanium found in nature. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Four artificial isotopes of titanium have also been made. These are all radioactive. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
None of the radioactive isotopes of titanium has any commercial applications.
Extraction
The methods used to obtain titanium are similar to those used for other
metals. One way to make the metal is to heat one of its compounds with
another metal, such as
magnesium:
Another approach is to pass an electric current through a molten (melted)
compound of titanium:
Uses
By far the most important use of titanium is in making alloys. The metal is most commonly added to steel. It adds strength to the steel and makes it more resistant to corrosion (rusting). Titanium also has another advantage in alloys. Its density is less than half that of steel. So a steel alloy containing titanium weighs less, pound-for-pound, than does the pure steel alloy.
These properties explain why titanium steel is so desirable for spacecraft and aircraft applications. In fact, about 65 percent of all titanium sold is used in aerospace applications. Titanium alloys are used in the airframes (bodies) and engines of aircraft and spacecraft. Other uses are in armored vehicles, armored vests, and helmets, in jewelry, eyeglasses, bicycles, golf clubs, and other sports equipment; in specialized dental implants; in power-generating plants and other types of factories; and in roofs, faces, columns, walls, ceilings, and other parts of buildings.
Titanium alloys have also become popular in body implants, such as artificial hips and knees. These alloys are light, strong, long-lasting, and biocompatible. Biocompatible means that the alloy does not cause a reaction when placed into the body.
Titanium tetrachloride combines with moisture in the air to form a dense white cloud. Skywriters use titanium tetrachloride to form letters in the sky.
Compounds
The most important compound of titanium is titanium dioxide (TiO 2 ). In 1996, 1,230,000 metric tons of this compound was produced in the United States. Titanium dioxide is a dense white powder with excellent hiding power. That term means that anything beneath it cannot be seen well. This property accounts for the major use of titanium dioxide: making white

About 40 percent of all titanium dioxide used in the United States goes into paper and plastic materials. Titanium dioxide gives "body" to paper and makes it opaque (unable to see through it). Other uses are in floor coverings, fabrics and textiles, ceramics, ink, roofing materials, and catalysts in industrial operations. A catalyst is a substance used to speed up or slow down a chemical reaction without undergoing any change itself.
Another interesting compound is titanium tetrachloride (TiCl 4 ). Titanium tetrachloride is a clear, colorless liquid when kept in a sealed container. However, it changes dramatically when exposed to the air. It combines with moisture in the air to form a dense white cloud. Skywriters use titanium tetrachloride to form letters in the sky. The compound is also used to make smokescreens. Smoke effects used in motion pictures and television programs sometimes are produced with titanium tetrachloride.
Health effects
Titanium appears to have no harmful effects on plants or humans. It has also not been shown to have any role in maintaining good health.
Is it from an encyclopedia?
It can be referenced as a primary electronic source/ website