LITHIUM

Overview
Lithium is the first member of the alkali metal family. The alkali metals are the elements that make up Group 1 (IA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. The alkali metals include sodium, potassium, rubidium, cesium, and rancium. Lithium is also the least dense of all metals. It has a density about half that of water.
Credit for the discovery of lithium usually goes to Swedish chemist Johan August Arfwedson (or Arfvedson; 1792-1841). Arfwedson found the new element in a mineral that had first been identified about twenty years earlier by Brazilian scientist Jozée Bonifácio de Andrada e Silva (1763-1838). That mineral, petalite, is still a major source of lithium today.
Lithium has a number of important and interesting uses. In recent years, it has been used to make lightweight, efficient batteries. Compounds of lithium have also been used to treat a mental disorder known as bipolar disorder.
SYMBOL
Li
ATOMIC NUMBER
3
ATOMIC MASS
6.941
FAMILY
Group 1 (IA)
Alkali metal
PRONUNCIATION
LI-thee-um
Discovery and naming
The first clues to the existence of lithium surfaced in 1800. De Andrada was a Brazilian scientist and statesman visiting in Scandinavia. During one of his trips to the countryside, he came across a mineral that he did not recognize. He called the mineral petalite.
Some scientists were not convinced that petalite was a new mineral. But in 1817, the same mineral was rediscovered on the island of Utö. Interest in the mineral grew.
Arfwedson was troubled by the results of his analysis of petalite. In his studies, he could not identify 10 percent of the mineral. He finally concluded that the missing 10 percent must be a new element. He called the new element lithium, from the Greek word lithos for "stone."
Arfwedson was not able to produce pure lithium. About a year later, however, Swedish chemist William Thomas Brande (1788-1866) and English chemist Sir Humphry Davy (1778-1829) were both able to extract the pure metal from its compounds. (See sidebar on Davy in the calcium entry in Volume 1.)
Physical properties
Lithium is a very soft, silvery metal. It has a melting point of 180.54°C (356.97°F) and a boiling point of about 1,335°C (2,435°F). Its density is 0.534 grams per cubic centimeter. By comparison, the density of water is 1.000 grams per cubic centimeter. Lithium's hardness on the Mohs scale is 0.6. The Mohs scale is a way of expressing the hardness of a material. It runs from 0 (for talc) to 10 (for diamond). A hardness of 0.6 means that the material can be scratched with a fingernail.
Chemical properties
Lithium is an active element, but not as active as the other alkali metals. It reacts slowly with water at room temperature and more rapidly at higher temperatures. It also reacts with most acids, giving off hydrogen gas. Lithium does not react with oxygen at room temperature, but above 100°C does so to form lithium oxide (Li 2 0). Under the proper conditions, the element also combines with sulfur, hydrogen, nitrogen, and the halogens.
Occurrence in nature
The abundance of lithium in the Earth's crust is estimated to be about 0.005 percent. That places it among the top 15 elements

The world's largest producer of lithium is the United States. Three of the largest U.S. mines are located in Silver Peak, Nevada, and Kings Mountain and Bessemer City, North Carolina. Other major producers of lithium compounds are Australia, Russia, Canada, Zimbabwe, Chile, and China.
Isotopes
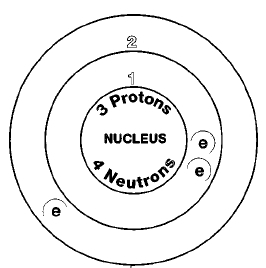
Two naturally occurring isotopes of lithium exist, Lithium-6 and lithium-7. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
In addition, three radioactive isotopes of lithium have been produced. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive. None of these isotopes has any important commercial application.
Extraction
Lithium compounds are first converted to lithium chloride (LiCl). Then, an
electric current is passed through molted (melted) lithium
chloride.
The current separates the compound into lithium and chlorine gas:
Uses and compounds
Lithium metal and its compounds have a great many uses. Two of the most significant applications are in the glass and ceramics field and in the production of aluminum. The addition of a small amount of lithium carbonate (Li 2 CO 3 ) to a glass or ceramic makes the material stronger. Examples of the use of lithium carbonate are shock-resistant cookware (such as the Pyrex brand) and black-and-white television tubes. About 40 percent of the lithium used in the United States in 1996 went to these applications.
Lithium carbonate is added to glass to make it stronger. Pyrex cookware is made up of this kind of glass.
Producers of aluminum also use lithium carbonate in preparing aluminum metal from aluminum oxide. Lithium carbonate reduces the heat needed to make the reaction occur. As a result, producers save money by using less energy. In 1996, about 20 percent of all lithium carbonate produced in the United States went to this application.
Another important compound of lithium is lithium stearate. Lithium stearate is added to petroleum to make a thick lubricating grease. The grease is used in many industrial applications because it does not break down at high temperatures, it does not become hard when cooled, and it does not react with water or oxygen in the air. Lithium greases are used in military, industrial, automotive, aircraft, and marine applications. Lithium stearate is also used as an additive in cosmetics and plastics. Overall, the manufacture of lithium stearate is the third most important use of lithium compounds after glasses and ceramics manufacture and aluminum production.
The first commercial use of lithium was in the production of alloys. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals. Early lithium alloys included lead and were used to make tough ball bearings for machinery.
Feeling better with lithium
A n exciting new use for lithium carbonate was discovered in 1949. John Cade (1912-80), an Australian physician, found that patients with bipolar disorder benefitted from taking lithium carbonate. Bipolar disorder is a condition once known as manic-depressive disorder. The condition is characterized by dramatic mood swings. A person can be very happy and carefree one moment, but terribly depressed the next moment. Some patients become so depressed that they commit suicide. Until 1949, there was no effective treatment for bipolar disorder.
Cade found that most patients who took lithium carbonate were relieved of at least some of their symptoms. Their "high" points were not as high, and their "low" points were not as low. The compound helped someone with bipolar disorder to live a quieter, more normal life. Today, more than 60 percent of those with bipolar disorder benefit from lithium treatments.
As with most medications, lithium compounds can have side effects. They can cause nausea, dizziness, diarrhea, dry mouth, and weight gain. But these side effects can usually be controlled. And they are often a small price to pay for relief from the terrible effects of bipolar disorder.
Today, the most commonly used alloys of lithium are made with aluminum or magnesium. These alloys are very light, but very strong. They are used for armor plates and in aerospace applications.
Lithium compounds are also used as catalysts in many different industrial processes. A catalyst is a substance used to speed up or slow down a chemical reaction. The catalyst does not undergo any change itself during the reaction. For example, one lithium catalyst is used to make tough, strong, synthetic (artificial) rubber. It does not have to be vulcanized (heat-treated) like natural rubber.
Lithium has become important in the manufacture of batteries. A battery is a device for converting chemical energy into electrical energy. Car batteries use a chemical reaction between lead and sulfuric acid to make electrical energy.
Lithium batteries are much lighter than lead and sulfuric acid batteries. They also reduce the use of toxic lead and cadmium. Lithium batteries are used in products such as watches, microcomputers, cameras, small appliances, electronic games, toys, and many kinds of military and space vehicles.
Lithium compounds tend to harm the kidneys.
Health effects
Lithium and its compounds have a range of effects on the human body. For instance, compounds of lithium tend to harm the kidneys. And lithium carbonate (Li 2 CO 3 ) can affect a person's mental health (see accompanying sidebar).
LCO (Lithium-Cobalt-Oxide) 39% of batteries produced 41kilo tonnes pa
Still one of the highest energy density chemistries, but expect to see only steady growth as automotive and utility scale applications grow
NCM (Nickel-Cobalt-Manganese) 22% 23ktpa
Of the 10 top selling Chinese EV’s using LFP chemistry, 6 are already converting to NCM
Experiencing fastest growth with a good mix of energy density, power, cost and safety for automotive applications; new chemistries constantly developing
NCA (Nickel-Cobalt-Aluminium) 11% 12ktpa
Extremely high energy density, power and manufacturing experience make it a good candidate for automotive, such as the A18650.
LMO (Lithium-Manganese-Oxide) 19% 20ktpa
Relatively low energy density (one-third of LCO), but the absence of cobalt makes this a low cost alternative cathode material
9% 9ktpa
Is battery size an issue? Obviosly weight is.
Most consumers are familiar with the omnipresent A, AA, and AAA battery sizes, but many of the same consumers do not understand the meaning of other battery size terms, such as the 18650. Manufacturers produce the lithium ion 18650 at a size of 18mm by 65 mm.
Please help me understanding it.