ARGON

Overview
Argon is a noble gas. The noble gases are the six elements in Group 18 (VIIIA) of the periodic table. The periodic table is a chart that shows how the chemical elements are related to each other. The noble gases are sometimes called inert gases because Group 18 (VIIIA) elements react with very few other elements. In fact, no compound of argon has ever been produced.
Argon was discovered in 1894 by English chemist John William Strutt, most commonly known as Lord Rayleigh (1842-1919), and Scottish chemist William Ramsay (1852-1916). It was the first of the noble gases to be isolated.
Rayleigh and Ramsay discovered argon by the fractional distillation of liquid air. Fractional distillation is the process of letting liquid air slowly warm up. As the air warms, different elements change from a liquid back to a gas. The portion of air that changes back to a gas at -185.86°C (-302.55°F) is argon.
SYMBOL
Ar
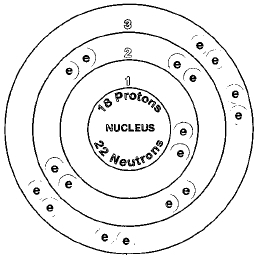
ATOMIC NUMBER
18
ATOMIC MASS
39.948
FAMILY
Group 18 (VIIIA) Noble gas
PRONUNCIATION
AR-gon
Argon is used to provide an inert blanket for certain industrial operations. An inert blanket of gas prevents any chemicals in the operation from reacting with oxygen and other substances present in air. Argon is also used in making "neon" lamps and in lasers.
Discovery and naming
Argon was discovered in 1894. However, English scientist Henry Cavendish (1731-1810) had predicted the existence of argon 200 years earlier. When Cavendish removed oxygen and nitrogen from air, he found that a very small amount of gas remained. He guessed that another element was in the air, but he was unable to identify what it was.
When Ramsay repeated Cavendish's experiments in the 1890s, he, too, found a tiny amount of unidentified gas in the air. But Ramsay had an advantage over Cavendish: he could use spectroscopy, which did not exist in Cavendish's time. Spectroscopy is the process of analyzing light produced when an element is heated. The spectrum (plural: spectra) of an element consists of a series of colored lines and is different for every element.
Ramsay studied the spectrum of the unidentified gas. He found a series of lines that did not belong to any other element. He was convinced that he had found a new element. Meanwhile, Rayleigh was doing similar work at almost the same time. He made his discovery at about the same time Ramsay did. The two scientists decided to make their announcement together. The name argon comes from the Greek word argos, "the lazy one." The name is based on argon's inability to react with anything.
The discovery of argon created a problem for chemists. It was the first noble gas to be discovered. Where should it go in the periodic table? At the time, the table ended with Group 17 (VIIA) at the right. Ramsay suggested that the periodic table might have to be extended. He proposed adding a whole new group to the table. That group would be placed to the right of Group 17 (VIIA).
Ramsay's suggestion was accepted, but it created an interesting new problem for chemists. If there was a new group in the periodic table, where were the other elements that belonged in the group?
Fortunately, chemists had a good idea what these missing elements might look like. All of the elements in a single group are very much like each other. Chemists looked for more inactive gases. Within the next five years, they had found the remaining members of the group: helium, krypton, neon, radon, and xenon.
The symbol A was used for argon until the 1950s when chemists agreed to use the two letter symbol Ar for the element.
Physical properties
Argon is a colorless, odorless, tasteless gas. Its density is 1.784 grams per liter. The density of air, for comparison, is about 1.29 grams per liter. Argon changes from a gas to a liquid at -185.86°C (-302.55°F). Then it changes from a liquid to a solid at -189.3°C (-308.7°F).
Chemical properties
Argon is chemically inactive. On rare occasions, and under extreme conditions, it forms weak, compound-like structures.
Occurrence in nature
The abundance of argon in the atmosphere is about 0.93 percent. It is also found in the Earth's crust to the extent of about 4 parts per million.
Extraction
Argon can be produced from liquid air by fractional distillation. It can
also be produced by heating nitrogen gas from the atmosphere with hot
magnesium
or
calcium.
The magnesium or calcium combines with nitrogen to form a nitride:
A little argon always occurs as an impurity with
nitrogen.
It remains behind because it does not react with magnesium or calcium.
Argon also occurs in wells with natural gas. When the natural gas is purified, some argon can be recovered as a by-product.
Isotopes
Three isotopes of argon exist naturally. They are argon-36, argon-38, and argon-40. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's
Six radioactive isotopes of argon are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
No radioactive isotopes of argon have any practical application. One non-radioactive isotope is used, however, to find the age of very old rocks. This method of dating rocks is described in the potassium entry.
Uses
Argon is used in situations where materials need to be protected from oxygen or other gases. A good example is an incandescent lightbulb, which consists of a metal wire inside a clear glass bulb. An electric current passes through the wire, causing it to get very hot and give off light.
Oxygen will combine with the hot metal very easily, forming a compound of the metal and oxygen. This compound will not conduct an electric current very well, thereby causing the lightbulb to stop giving off light.
Argon, however, is used to prevent this from happening. Because argon is inert, it will not react with the hot wire, leaving the metal hot for very long periods of time. The lightbulb will stop giving off light only when the metal breaks. Then it can no longer carry an electric current.
Argon is also used in welding. Welding is the process by which two metals are joined to each other. In most cases, the two metals are heated to very high temperatures. As they get hot, they melt together.
However, as the metals get hot, they begin to react with oxygen. In this reaction, a compound of metal and oxygen is formed. It becomes very difficult to join the two metals if they have formed compounds, but introducing argon into the welding environment improves the bond.
Argon is also used in argon lasers and argon-dye lasers. A laser is a device that produces very bright light of a single color (frequency). An argon laser is used to treat skin conditions. The laser shines a blue-green light on the affected area of the skin. The energy from the laser is absorbed by hemoglobin and converted to heat. (Hemoglobin is the protein pigment in red blood cells. It transports oxygen to the tissues and carbon dioxide from them.) The blood vessels are damaged, but then sealed, prompting them to decompose and be reabsorbed into the body. Unwanted growths are flattened and dark spots are Lightened, with only a small risk of scarring.
An argon-dye laser is used in eye surgery. The color of light produced by the laser can be adjusted with high precision. It can be made to produce light ranging across the green-to-blue color range. Each shade of green or blue has a slightly different frequency. It can penetrate more or less deeply in the eye. The laser can be adjusted to treat a very specific part of the eye. The argon dye is used to treat tumors, damaged blood vessels, conditions involving the retina, and other kinds of eye problems.
Compounds
No compound of argon has ever been produced.
Health effects
Argon is not known to have any positive or negative effects on the health of plants or animals.