ANTIMONY

Overview
Antimony compounds have been used by humans for centuries. Women of ancient Egypt used stibic stone, antimony sulfide, (Sb 2 S 3 ), to darken their eyes. Antimony was also used in making colored glazes for beads and glassware. The chemical symbol for antimony was taken from the ancient name for the element, stibium. Not recognized as a chemical element until the Middle Ages, antimony became a common material used by alchemists.
Alchemy was a kind of pre-science that existed from about 500 B.C. to about the end of the 16th century. Alchemists wanted to find a way of changing lead, iron, and other metals into gold. They also wanted to find a way of having eternal life. Alchemy contained too much magic and mysticism to be a real science, but alchemists developed a number of techniques and produced many new materials that were later found to be useful in modern chemistry. Antimony was one of these materials.
SYMBOL
Sb
ATOMIC
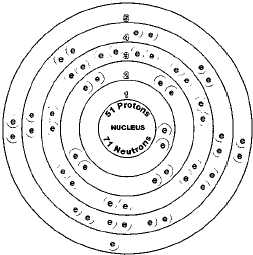
NUMBER 51
ATOMIC MASS
121.75
FAMILY
Group 15 (VA)
Nitrogen
PRONUNCIATION
AN-ti-moh-nee
Antimony is a metalloid. A metalloid is an element that has characteristics of both metals and non-metals. The metalloids can be found on either side of the staircase line on the right side of the periodic table (with the exception of aluminum, which is not considered a metalloid).
Antimony is primarily used in alloys, ceramics and glass, plastics, and flame retardant materials. Flame retardant materials do not burn with an open flame. Instead, they smolder or do not burn at all.
Discovery and naming
Compounds of antimony were known to ancient cultures. They have been found, for example, in the colored glazes used on beads, vases, and other glassware. But these compounds were not widely used until the Middle Ages when they became popular among alchemists. They thought that antimony could be used to convert lead into gold. It was during this period that records about the properties of antimony begin to appear.
The element was probably first named by Roman scholar Pliny ( A.D. 23-79), who called it stibium. Muslim alchemist Abu Musa Jabir Ibn Hayyan (c. 721-c. 815) probably first called it antimony— anti ("not") and monos ("alone"). The name comes from the fact that antimony does not occur alone in nature.
Alchemists used secret codes to write about much of their work, so modern scholars do not know a great deal about how antimony was used. The first detailed reports about antimony were published in 1707 when French chemist Nicolas Lemery (1645-1715) published his famous book, Treatise on Antimony.
Physical properties
Antimony is a silvery-white, shiny element that looks like a metal. It has a scaly surface and is hard and brittle like a non-metal. It can also be prepared as a black powder with a shiny brilliance to it.
The melting point of antimony is 630°C (1,170°F) and its boiling point is 1,635°C (2,980°F). It is a relatively soft material that can be scratched by glass. Its density is 6.68 grams per cubic centimeter.
A metalloid is an element that has characteristics of both metals and non-metals.
Chemical properties
Antimony is a moderately active element. It does not combine with oxygen in the air at room temperature. It also does not react with cold water or with most cold acids. It does dissolve in some hot acids, however, and in aqua regia. Aqua regia is a mixture of hydrochloric and nitric acids. It often reacts with materials that do not react with either acid separately.
Occurrence in nature
Antimony is rarely found in its native (as an element) state. Instead, it usually occurs as a compound. The most common minerals of antimony are stibnite, tetrahedrite, bournonite, boulangerite, and jamesonite. In most of these minerals, antimony is combined with sulfur to produce some form of antimony sulfide (Sb 2 S 3 ).
The largest producers of antimony are China, Russia, Bolivia, South Africa, and Kyrgyzstan, in that order. The United States produces antimony as a by-product at only one silver mine in Idaho.
The abundance of antimony is estimated to be about 0.2 parts per million, placing it in the bottom fifth among the chemical elements found in the Earth's crust. It is more abundant than silver or mercury, but less abundant than iodine.
Isotopes
There are two naturally occurring isotopes of antimony, antimony-121 and antimony-123. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
About 20 radioactive isotopes of antimony are also known. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
Two of antimony's radioactive isotopes are used commercially as tracers. These isotopes are antimony-124 and antimony-125. A tracer is an isotope injected into a living or non-living system. The movement of the isotope can then be followed as it moves through the system. For example, a small amount of antimony-124 could be injected into an oil pipeline. The presence of the isotope can be detected by means of an instrument held above the pipeline. The radiation given off by the isotope causes a light to flash or a sound to occur in the
Extraction
Antimony can be recovered from stibnite with hot iron:
About half the antimony produced in the United States is recycled from
old lead storage batteries used in cars and trucks.
Uses
Antimony is used to make alloys with a number of different metals. An alloy is made by melting and mixing two or more metals. The properties of the mixture are different than those of the individual metals. One of the most common of these

Other minor uses of antimony include the manufacture of glass and ceramics and the production of plastics. In glass and ceramics, a small amount of antimony insures that the final product will be clear and colorless. In the production of plastics, antimony is used as a catalyst. A catalyst is a substance used to speed up or slow down a chemical reaction. The catalyst does not undergo any change itself during the reaction.
Compounds
The most important use of antimony is in making compounds used in the manufacture of flame-retardant materials. Slightly more than half of all antimony goes to this use. These include antimony oxychloride (SbOCl), antimony pentoxide (Sb 2 O 5 ), antimony trichloride (SbCl 3 ), and antimony trioxide (Sb 2 O 3 ). These compounds are sprayed on or added to a fabric to make it flame retardant.
Health effects
Antimony and its compounds are dangerous to human health. In low levels, these materials can irritate the eyes and lungs. They may also cause stomach pain, diarrhea, vomiting, and stomach ulcers. At higher doses, antimony and its compounds can cause lung, heart, liver, and kidney damage. At very high doses, they can cause death.