Gardening
A successful garden represents a broad spectrum of chemical processes. Photosynthesis provides the route by which diverse chemical transformations use sunlight, water, carbon dioxide, and inorganic chemical elements to produce life-sustaining organic molecules and oxygen (Equation 1). This solarpowered rearrangement of matter is the foundation of almost all ecosystems and is an important example of how chemistry applies to the study of life.
(1)
The light reactions that occur in the chloroplasts, in addition to catalyzing the evolution of oxygen, also produce ATP and NADPH. ATP energy drives the endergonic reactions, and NADPH drives the reducing power required to bind the hydrogen ions to carbon atoms, and thus to synthesize all the organic compounds necessary for the growth and development of plants in a garden. Plants retrieve the needed ATP energy and reducing power by stripping away the energetic electrons and using them to make the high-energy compound ATP. This is possible because the electrons still carry the energy contributed by their encounter with the photon of light. When electrons are removed from chemical bonds, the food molecules are oxidized (i.e., lose electrons). Under aerobic conditions, the electrons that are harvested (as ATP is being formed) are eventually donated to oxygen gas, in a process known as cellular respiration (Equation 2).
(2)
Plants (including algae) are uniquely self-sufficient in that they are able to harvest and transform radiant energy into the chemical energy required to transform chemically simple molecules and elements (CO 2 , H 2 O, and nutrient elements) into substances (including carbohydrates, fats, proteins, alcohols, and hormones) needed for the garden plants to complete their life cycle, and to produce harvestable products.
Chemical processes that occur in the soil and aerial environment determine the extent and rate of all plant metabolic processes. The crop growth factors—water, light, essential nutrient elements (Table 1), temperature, and space—are utilized most efficiently when the chemical, physical, and microbiological interactions among the crops, soil, and air are optimal.
Almost all physiological processes in plants take place in the presence of water. Essential anabolic reactions (photosynthesis, assimilation, and protein synthesis), and catabolic ones (respiration and hydrolysis) occur in an aqueous cellular environment. Essential elements absorbed by plant roots, and the foods and other metabolites manufactured by the leaves and other tissues, move in aqueous solution from the regions of absorption or manufacture to other parts of the plant where additional anabolic reactions and ultimate food storage take place. Water is the major constituent of protoplasm, and is particularly abundant in young and growing tissues.
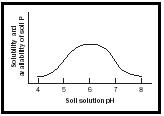
Good soil quality is essential for the crop's root system to function properly, and to ensure that all added chemicals are used efficiently. Incorporating into the soil the required amounts of lime (for soil pH adjustment) and fertilizer (all nutrients needed to amend the soil) is an important first step toward improving soil quality and creating a suitable rooting environment for crops. Deep placement of phosphorus in the soil is beneficial for several reasons. This element is sparingly soluble in the water present in the soil, especially when the soil is cold and/or when the soil pH is not properly adjusted (see Figure 1), and therefore phosphorus diffuses slowly to the site of uptake by roots. Phosphorus is the first essential mineral element the newly growing seedling requires from soil after the seed reserves (phytic acid) for this element are exhausted.
A healthy root system functions to absorb water and dissolved chemicals and translocate them to the above-ground tissues. Many crop growth regulators, hormones, and other chemicals crucial to providing biotic and
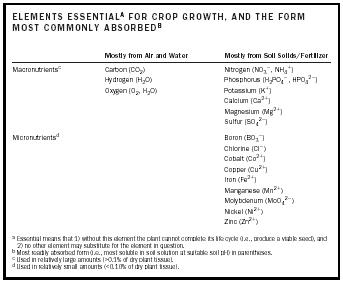
| ELEMENTS ESSENTIAL A FOR CROP GROWTH, AND THE FORM MOST COMMONLY ABSORBED B | ||
| Mostly from Air and Water | Mostly from Soil Solids/Fertilizer | |
|
a
Essential means that 1) without this element the plant cannot
complete its life cycle (i.e., produce a viable seed), and
2) no other element may substitute for the element in question. |
||
| b Most readily absorbed form (i.e., most soluble in soil solution at suitable soil pH) in parentheses. | ||
| c Used in relatively large amounts (>0.1% of dry plant tissue). | ||
| d Used in relatively small amounts (<0.10% of dry plant tissue). | ||
| Macronutrients c | Carbon (CO 2 ) | Nitrogen (NO 3 − , NH 4 + ) |
| Hydrogen (H 2 O) | Phosphorus (H 2 PO 4 − , HPO 4 2− ) | |
| Oxygen (O 2 , H 2 O) | Potassium(K + ) | |
| Calcium (Ca 2+ ) | ||
| Magnesium (Mg 2+ ) | ||
| Sulfur (SO 4 2− ) | ||
| Micronutrients d | Boron (BO 3 − ) | |
| Chlorine (Cl − ) | ||
| Cobalt (Co 2+ ) | ||
| Copper (Cu 2+ ) | ||
| Iron (Fe 2+ ) | ||
| Manganese (Mn 2+ ) | ||
| Molybdenum (MoO 4 2− ) | ||
| Nickel (Ni 2+ ) | ||
| Zinc (Zn 2+ ) | ||
abiotic stress tolerance to the crop are synthesized in roots. Many of these chemical substances are translocated from root to shoot tissues in the same part of the vascular system (xylem tissue) as is the water, dissolved nutrient elements, and soil-applied pesticides. Some of the root-synthesized organic chemicals (such as the hormone abscisic acid) have a profound impact on the structure and function of above-ground tissues (leaf and reproductive tissue abscission and stomatal control of gaseous exchange, for example).
A healthy root system also will secrete organic chemicals (such as sugars, organic acids, and amino acids) into a cylindrical soil zone around the root system that is relatively rich in heterotrophic soil organisms. These soil microbes use these chemicals as energy and carbon sources for their own growth and reproduction, and catalyze many soil chemical transformations that are vital to the garden. With suitable soil moisture, temperature, and aeration, and in the presence of the appropriate microbes, a portion of the immobilized N, P, and S is converted to the inorganic form, and thus becomes available for plant uptake. Many of the essential elements cycle between organic and inorganic forms continuously throughout the growing season.
The key to ensuring that garden soil is in a proper chemical condition is to make certain that the soil pH and nutrient element status are correct for the crops of interest. Each crop has one pH value at which it grows best. Normally, the higher the amount of organic matter in the soil, the lower is the ideal soil pH.
Soil acidity commonly is decreased by supplying carbonates, oxides, or hydroxides of calcium and magnesium, compounds that are referred to as agricultural limes. Wood ashes (as from a fireplace) also are used to help raise soil pH. The primary sources of carbonates, and by far the most commonly used of all liming materials, are calcite, mostly CaCO 3 , and dolomite, primarily [CaMg(CO 3 ) 2 ]. Given the fact that the balance of nutrients in plant tissues (the balance of N-P-K-Ca-Mg-S, for example) is even more important than the absolute amount of each nutrient present. Dolomite usually is the preferred liming material, because it supplies relatively equivalent quantities of two essential nutrients. Other sources of carbonates, such as marl, oyster shells, basic slag, and precipitated carbohydrates, all of which are relatively slow-acting, are also used to help control acidity.
Two additional sources of lime are noteworthy, especially when a rapid change in soil pH is desired. Calcium oxide (CaO), called quicklime or burned lime, and calcium hydroxide [Ca(OH) 2 ], called hydrated lime, are more irritating to handle, and more expensive, than is limestone, but are sometimes favored by gardeners who desire to adjust soil pH quickly.
All liming materials, whether oxide, hydroxide, or carbonate, react with soil water and carbon dioxide to yield the bicarbonate form when applied to acid soil. The partial pressure of carbon dioxide in the soil usually is several hundred times greater than that in atmospheric air, and drives the reaction that produces Ca(HCO 3 ) 2 , which is very important in buffering the soil solution (see Equation 3).
(3)
Two attributes are required of any liming material: 1) a cation capable of displacing soil colloid-adsorbed H + and Al +++ (also a source of soil acidity); and 2) an anion capable of neutralizing the displaced H + and Al +++ (see Equation 4).
(4)
The insolubility of Al(OH) 3 and the diffusion of CO 2 to the atmosphere drive this reaction to completion. Also, adsorption of cations onto the colloid complex raises the percentage base saturation (extent to which the colloidal complex is saturated with exchangeable cations other than hydrogen and aluminum, expressed as a percentage of the total cation exchange capacity) of the colloidal complex, increasing the pH of the soil solution accordingly.
Soil with an appropriate pH for the crops being grown will provide the essential nutrient elements in the most soluble form, and also will retain these elements in the effective rooting zone most fully, because the nutrients are more likely to be chemically adsorbed on the colloidal exchange complex, and less subject to leaching loss. One major problem resulting from raising the soil pH above about pH 6.5–6.7 is that several of the essential micronutrient elements begin to precipitate out of solution, becoming unavailable for uptake. Soils high in clay and/or organic matter are much harder to overlime, and generally require a slightly lower pH for optimal crop growth.
The vast majority of crops normally grown in most gardens are healthiest when the mineral soil pH is in the 5.8–6.2 range. These crops grow equally well in soils fairly high in organic matter (2-5%) at a somewhat lower pH, in the 5.2–5.6 range.

The decomposition of organic matter in the soil, and the reaction of NH 4 + -containing fertilizer materials in soil solution, contribute to an increase in soil acidity, especially when the soil is well-aerated and warm, and when the right kinds of bacteria are present (Equation 5). The process begins with the microbially-induced mineralization of nitrogen from organic to the NH 4 + form, followed by a two-step nitrification process. In the first step, obligate autotrophic bacteria ( Nitrosomonas spp. ), that obtain their energy from the oxidation of nitrogen and their carbon from CO 2, oxidize NH 4 + to NO 2 − . In the second reaction, NO 2 − is further oxidized to NO 3 − in the presence of autotrophic bacteria ( Nitrobacter spp. ). Under certain conditions other bacteria can be involved in both nitrification steps. The reaction rates associated with nitrification in most well-drained soils are NO 2 − to NO 3 − > NH 4 + to NO 2 − . As a result, NO 2 − generally does not accumulate in soils, which is fortunate, because this ion is toxic to plant roots. The nitrate ion is more mobile than is the ammonium ion, and therefore much more highly leachable. Factors promoting nitrification in soils include 1) supply of ammonium, 2) population of nitrifying organisms, 3) soil pH, 4) soil aeration, 5) soil moisture, and 6) temperature. Because nitrification is suppressed in a cold soil, ammonium-containing fertilizers applied in the late fall or winter promote accumulation of ammonium rather than nitrate, and thus nitrogen is retained in the garden soil rather than being lost through leaching. Under wet soil conditions, and in the presence of anaerobic bacteria, the nitrate ion can be lost through denitrification to N 2 , NO x and N 2 O, which is a greenhouse gas, and thus potentially a contributor to global warming (see Equation 5).
(5)
One of the greatest values of a properly limed and pH-adjusted soil is that applied fertilizer and pesticide materials are much more likely to remain in

the soil. This practice leads to the retention of more fertilizer elements in the effective rooting zone during periods of heavy rain. A higher proportion of a properly-limed soil colloid's cation exchange capacity is comprised of basic ions, such as calcium and magnesium, rather than hydrogen ions, thus reducing the potential for leaching loss of essential elements. Considering the relative strength of cation adsorption on the surface of soil colloids (both inorganic and organic), hydrogen ions are held the strongest, followed by aluminum, calcium, magnesium, potassium/ammonium, and finally sodium ions (held least tightly). The more fully the cation exchange capacity of a soil is satisfied by calcium and/or magnesium (i.e., the better limed it is), the lower is the leaching potential of applied nutrient elements such as potassium.
Another important reason for maintaining soil pH at the proper level is that most of the soil microorganisms that benefit the garden in so many ways (for example, decaying plant residues that otherwise would serve as a haven for pathogens and insects) function best in a well-limed soil. The bacteria that are able to produce nodules (tumor-like growths on the roots that serve as the site for symbiotic biological nitrogen fixation) on the roots of legume crops, for example, function best at pH 6.3–6.5. When the legume crop is healthy, and the proper strains of symbiotic bacteria are present (each legume requires its own bacterial strains), the nitrogenase enzyme (provided by the bacteria) within the nodule catalyzes the reduction of diatomic nitrogen gas to the ammonium form of nitrogen, one of the two forms of nitrogen (nitrate being the other) plants can readily use. Since the heterotrophic bacteria obtain their required energy (of ATP) from the host plant, this association is a true symbiosis. The legume plant dedicates about 14–18 moles of ATP for each mole of nitrogen gas reduced. When a legume crop is planted, and when this symbiotic relationship is functioning properly, no synthetic nitrogen fertilizer needs to be applied, and subsequent nonlegume crops planted in the same area will benefit from the residual nitrogen ultimately returned to the soil from the legume crop's residues.
A healthy garden represents a chemical laboratory in which a host of chemical processes are occurring in synchrony with our natural world. When the gardener has chosen adapted crop varieties, and has managed both soil and crops wisely, these chemical processes unfold in such a way that the garden harvests the sun's energy efficiently, and converts a portion into useful products.
SEE ALSO Agricultural Chemistry ; Herbicides ; Insecticides ; Pesticides .
Robert P. Patterson
Donald E. Moreland