Agricultural Chemistry
Agricultural chemistry must be considered within the context of the soil ecosystem in which living and nonliving components interact in complicated cycles that are critical to all living things. Carbon inputs from photosynthetic organisms ultimately provide the fuel for many soil organisms to grow and reproduce. Soil organisms, in turn, promote organic carbon degradation and catalyze the release of nutrients required for plant growth. The stability and productivity of agricultural ecosystems rely on efficient functioning of these and other processes, whereby carbon and nutrients such as nitrogen and phosphorus are recycled. Human-induced perturbations to the system, such as those that occur with pesticide or fertilizer application, alter ecosystem processes, sometimes with negative environmental consequences.
Inorganic Components of the Agricultural Ecosystem
Soil is the primary medium in which biological activity and chemical reactions occur. It is a three- phase system consisting of solid, liquid, and gas. Approximately 50 percent of the volume in a typical agricultural soil is solid material classified chemically as either organic or inorganic compounds. Organic materials usually constitute 1 to 5 percent of the weight of the solid phase. The remainder of the soil volume is pore space that is either filled with gases such as CO 2 and O 2 , or water.
Surface area and charge characteristics of the inorganic portion of the solid phase control chemical reactivity. Soil particles are classified based on their size, with sand-sized particles having diameters of 2 to 0.05 millimeters (0.08 to 0.002 inches) and silt-sized particles from 0.05 to 0.002 millimeters (0.002 to 0.00008 inches). Clay-sized materials of less than 0.002 millimeters (0.00008 inches) in diameter have the largest surface area per unit weight, reaching as much as 800 meters (2,625 feet) squared per gram. Because of large surface areas, clay-sized materials greatly influence the sorption of chemicals such as fertilizers and pesticides and play a major role in catalyzing reactions.
Crystalline layer silicates or phyllosilicates present in the clay-sized fraction are especially important because they function as ion exchangers. Most phyllosilicates have a net negative charge and thus attract cations. This cation exchange capacity (CEC) controls whether plant nutrients, pesticides, and other charged molecules are retained in soil or if they are transported out of the soil system. In contrast, aluminum and iron oxides also present in the clay-sized fraction typically possess a net positive charge or an anion exchange capacity (AEC). Soils in temperate regions are dominated most often by solid phase materials that impart a net CEC, whereas soils in tropical regions often contain oxides that contribute substantial AEC.
Organic Components of the Agricultural Ecosystem
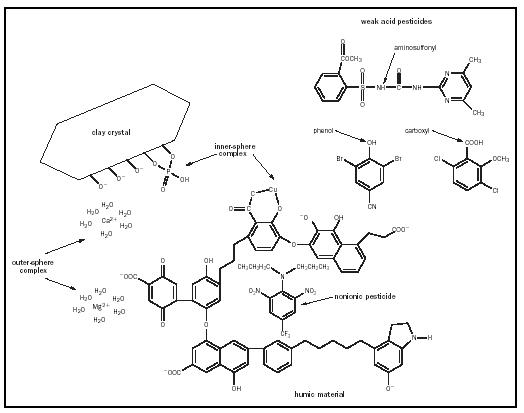
Organic materials contained within the solid phase, although only a small percentage of the total soil weight, are extremely important in controlling chemical and physical processes in soil. Organic matter exists in the form of recognizable molecules such as proteins and organic acids, and in large polymers called humic materials or humus. Humus is dominated by acidic functional groups (−OH and −COOH) capable of developing a negative charge and contributing substantial CEC. These large polymers possess a three-dimensional conformation that creates hydrophobic regions important in retaining nonionic synthetic organic compounds such as pesticides. Nonionic pesticides partition into these hydrophobic regions, thereby decreasing off-site movement and biological availability (see Figure 1).
A wide variety of organisms live in soil, including microorganisms not visible to the naked eye such as bacteria, fungi, protozoa, some algae, and viruses. Bacteria are present in the largest numbers, but fungi produce more biomass per unit weight of soil than any other group of microorganisms. Much of agricultural chemistry as it relates to nutrient cycles, pesticide transformation, plant growth, and organic matter degradation involves the participation of microorganisms. Microorganisms produce both intracellular and extracellular enzymes that increase reaction rates, oxidize and reduce organic and inorganic compounds, and synthesize organic molecules that modify soil chemical and physical properties.
Additional organisms in soil such as insects, nematodes, and earthworms also alter the soil ecosystem in a manner that directly or indirectly affects chemical reactions. These organisms physically process plant-derived organic materials prior to biochemical degradation by microorganisms. Nutrient release from organic materials is thus accelerated because the meso- and macrofauna expose more organic matter surface area to microbial breakdown and redistribute such materials in soil to areas of intense microbial activity.
In addition, bioturbation may also cause physical changes to the soil structure that increase pore space or modify water movement. Changes in O 2 concentration or soil water content will control biotic and abiotic reactions, altering rates of nutrient cycling and organic matter degradation.
Plant roots also modify soil by producing a zone of intense biological activity called the rhizosphere. This is a region of soil influenced by the root, most often delineated by comparing microbial numbers at greater distance from the root surface. Carbon compounds exuded or sloughed off from roots are used as a food source by microorganisms, thereby causing increased growth and activity. Microbial numbers above those of the bulk soil, which displays no root influence, indicate that the rhizosphere extends to 5 millimeters (0.2 inches) or less. Rhizosphere microorganisms that capitalize on carbon from the plant root interact physically and biochemically with the root, potentially producing positive or negative effects on plant growth.
Soil Chemistry
Biological availabilities and transport phenomena of ions and molecules in soil are controlled by the type of bonding that occurs with the solid phase. Ions such as those typically formed when amending soils with inorganic fertilizers interact with high surface area clay and humic colloids to form either outer- or inner-sphere complexes (see Figure 1). Outer-sphere complexes result when ions, electrostatically attracted to an oppositely charged colloidal surface, retain their shell of hydrating water molecules. These loosely held ions satisfy the excess positive or negative charge of the colloid, but are separated from the colloid's surface by one or more layers of water. In contrast, inner-sphere complexes form when the ion loses its hydration water to form a much stronger covalent bond with the colloid. Nutrient ions held in outer-sphere complexes are plant-available because they may be exchanged with ions of the same charge, but nutrients held by an inner-sphere mechanism are not available until the covalent bond is broken.
Most soils contain a net CEC often reported in centimoles of charge per kilogram of soil (cmol c /kg). Biological and physical characteristics of the soil are controlled by the amount of CEC and the specific cations involved. Soils dominated by high surface area clays or humus display the highest CECs, whereas soils with large amounts of sand or silt, and only small amounts of humus, exhibit much lower CECs. Highly charged cations with small hydrated radii such as Al 3+ are more tightly held on the CEC and less likely to exchange than larger, less highly charged cations such as Na + . This general relationship is superseded when a specific inner-sphere complex forms such as between Cu 2+ and humus, or K + and clay. An even more dramatic example is that of two plant nutrients, NO 3 − and PO 4 3− . Negatively charged NO 3 − readily leaches out of soil, but PO 4 3 − is retained quite strongly because it forms an inner-sphere complex (see Figure 1).
The percentage of the CEC occupied by specific cations influences soil pH and associated characteristics relevant to plant growth and soil biological activity. Only the most strongly held cations remain in soils in high rainfall areas. Al 3+ dominates the CEC, hydrolyzing when released from the solid phase to the soil solution to form acidic soils with pH values often below 5.
Al 3+ + H 2 O ↔ AlOH 2+ + H +
In contrast, soils located in lower rainfall areas accumulate less tightly bound cations such as Ca 2+ , Mg 2+ , K + , and Na + and have higher pH values between 5 and 7. In the most arid regions, large amounts of OH − -generating sodium and calcium salts accumulate, causing soil pH values to exceed 7. Plant growth is optimal in soils having pH values between 5.5 and 6.5 because aluminum toxicity occurring at lower pH values, and nutrient limitations caused by higher pH values, are avoided.
Soil Microbiology and Biochemistry
Biochemical transformations catalyzed largely by microorganisms are required for the sustained productivity of all ecosystems. Nutrients sequestered in organic materials and added in the form of fertilizers are cycled by microorganisms

in their quest for energy, reducing equivalents, and carbon. Microorganisms grow and reproduce by oxidizing organic or inorganic materials, thereby releasing electrons. The electrons are passed down a series of carriers aligned in a thermodynamic gradient designed to capture energy in the form of adenosine triphosphate (ATP) . Additional electrons originating from organic or inorganic materials are used to provide reducing equivalents necessary for synthesizing cell constituents. Carbon for cell growth is obtained from the organic materials being oxidized or captured in the form of CO 2 if inorganic materials are being oxidized.
Reduction- oxidation processes are therefore central to agricultural chemistry because oxidation of the electron source and reduction of the electron sink profoundly modify the respective element's chemical characteristics, and thus its behavior and biological availability in the environment. For example, microbial oxidation processes convert organic compounds to CO 2 , a gas, and NH 4 + , a cation, to NO 3 − , an anion. Electrons obtained in these oxidations are passed on to a terminal electron acceptor. Microorganisms use terminal electron acceptors in a sequence that maximizes energy yield starting with O 2 and proceeding through NO 3 − , Mn 4 + , Fe 3+ , SO 4 2− , and finally CO 2 , which upon reduction yield H 2 O, N 2 , Mn 2+ , Fe 2+ , H 2 S, and CH 4 , respectively.
Human Manipulation of Agricultural Ecosystems
Food and fiber production are typically optimized by carefully managing the agricultural ecosystem. Synthetic organic compounds are often applied

to control plant pests including weeds, insects, nematodes, and fungal pathogens. Pesticide fate is controlled by sorption to the solid phase and degradation rate. Because most soils have a CEC, cationic pesticides are so strongly held by soil that they are typically biologically unavailable. Weak acid pesticides containing carboxyl, phenolic hydroxyls, or aminosulfonyl functional groups are weakly retained by soil and thus most likely to leach or move off-site (see Figure 1). Weak bases, which may exist as positively charged or uncharged molecules, and nonionic compounds, are intermediate in their susceptibility to move off-site and cause environmental contamination. However, rapid degradation of some pesticides to form benign products eliminates the time available for transport, decreasing the potential for environmental problems. Both biotic and abiotic mechanisms catalyze degradative reactions, the rate of which is controlled by the pesticide's chemical structure.
With the advent of molecular techniques and the ability to transfer genes, an additional area of concern has emerged: the introduction of foreign genes into plant species for enhanced crop productivity. In addition, we have the ability to produce a variety of pharmaceutical chemicals in genetically modified plants using what has been termed "pharm crops." David Suzuki and Holly Dressel in From Naked Ape to Superspecies have commented on such genetic manipulations, addressing the risks of placing genes from one species into another. Not only is direct gene transfer from one living organism to another possible, but extracellular DNA preserved in soil systems is also potentially available for transfer, further increasing environmental risks.
Agricultural chemistry is most often linked to food and fiber production, specifically for human consumption. Jared Diamond in Guns, Germs, and Steel argues quite convincingly that it was our ability to domesticate crops and eliminate the need for hunting and gathering that allowed for the establishment of permanent settlements and the development of technologically advanced societies. The ensuing increase in human population has led to tremendous pressure to produce additional food from finite resources. Increased agricultural production, in combination with additional resource consumption and waste generation, has caused environmental degradation. By understanding key concepts in agricultural chemistry, we can utilize the soil resource to produce an adequate food supply and protect the environment.
SEE ALSO Fertilizer ; Herbicides ; Insecticides .
Matthew J. Morra
Bibliography
Brady, Nyle C., and Weil, Ray R. (2002). The Nature and Properties of Soils , 13th edition. Upper Saddle River, NJ: Prentice Hall.
Diamond, Jared M. (1997). Guns, Germs, and Steel: The Fates of Human Societies. New York: Norton.
Hillel, Daniel (1991). Out of the Earth: Civilization and the Life of the Soil. New York: Free Press.
Suzuki, David, and Dressel, Holly (1999). From Naked Ape to Superspecies: A Personal Perspective on Humanity and the Global Eco-crisis. New York: Stoddart.
Sylvia, David M.; Fuhrmann, Jeffry J.; Hartel, Peter G.; and Zuberer, David A., eds. (1998). Principles and Applications of Soil Microbiology. Upper Saddle River, NJ: Prentice Hall.
Comment about this article, ask questions, or add new information about this topic: