Fossil Fuels

Since the beginning of the industrial revolution , fossil fuels have been important sources of energy. European industrialization began in the late 1700s in England, and coal soon became a major fuel. In 1850 wood was still the main energy source in the United States. During the latter half of the nineteenth century, the United States and other industrialized nations relied on coal (a fossil fuel) to provide the energy for industrialization. Coal remained the major fuel source for many years, and then, in the latter half of the twentieth century, oil and natural gas became the primary energy sources. The first oil well was drilled in Pennsylvania in 1859.
In 2000, fossil fuels accounted for almost 90 percent of the world's energy production (see Table 1). Nuclear power and hydroelectric plants supplied about 13 percent and geochemical, wind, and solar energy sources supplied only a fraction of 1 percent. Biomass , including the burning of wood, is not included in the table because it is so difficult to estimate.
Although coal combustion produces substantially greater air pollution problems than does oil or natural gas combustion, because of its great abundance in the United States and other countries (such as Russia), there has been renewed interest in developing technology to burn coal more cleanly. However, all fossil fuels consist mainly of hydrocarbons (compounds that contain only carbon and hydrogen), which, upon complete combustion, yield carbon dioxide, a major greenhouse gas.
It is widely accepted in the scientific community that fossil fuels (coal, oil, and gas) have a biological origin and are ultimately derived from the buried remains of plant and animal matter, although some still argue in favor of a nonbiological or inorganic source. It is believed that a small fraction (much less than 1%) of dead plant and animal matter accumulates as deposited matter, is removed from contact with atmospheric oxygen, is subject to elevated temperatures and pressures (inhibiting decomposition by bacteria), and over geological time, is transformed into fossil fuels.
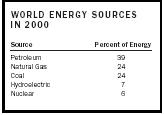
| WORLD ENERGY SOURCES IN 2000 | |
| Source | Percent of Energy |
| Petroleum | 39 |
| Natural Gas | 24 |
| Coal | 24 |
| Hydroelectric | 7 |
| Nuclear | 6 |
Coal
Coal is considered the remnant of plants that grew in swamps hundreds of millions of years ago, and thus its source is often characterized as terrestrial, signifying its association with continental land masses. Terrestrial plant material characteristically contains lignin, a carbon-based natural polymer that provides rigidity to nonaquatic plants and enables them to stand upright against the pull of gravity. Lignin is much more resistant to bacterial degradation than other botanical components, such as cellulose, and is considered a significant contributor to the chemical composition of coal.
The extent to which lignin and other plant matter has been metamorphosed by the high temperatures and pressures associated with the gradual burial of this material determines the grade of the coal produced. As the process of coal formation (coalification) proceeds, the product is increasingly characterized by lower moisture content, greater carbon and energy content, and a greater hardness. Lignite is the softest and least metamorphosed type of coal, with a relatively high moisture content, a low fixed carbon (nonvolatile carbon) content, and a low energy content. Subbituminous coal is the next highest grade, and upon further coalification it can be transformed to bituminous coal, or ultimately to anthracite. Anthracite is the hardest coal, possessing about 95 percent fixed carbon, the lowest moisture content, and the best energy content. Coals from different sources also contain differing amounts of inorganic mineral matter (ash), which remains as a residue upon burning and thus lowers the energy content of the coal. Table 2 compares the compositions of the various types of coal.
One mineral often associated with coal is pyrite, FeS 2 . The burning of coal contributes to pollution of the atmosphere, owing to the presence in coal of pyrite and organic sulfur-containing compounds. Coal is commonly burned in power plants that generate electricity, and both the inorganic (pyrite-containing) and organic forms of coal are oxidized to yield sulfur dioxide (SO 2 ). Sulfur dioxide reacts in air to form sulfuric acid (H 2 SO 4 ), which is a major cause of acid rain . Sulfuric acid and sulfur dioxide are also lung irritants, and thus health hazards, and contribute to the corrosion of structures by their acidification of all forms of precipitation (rain, snow, fog, sleet). The impact of the atmospheric precipitation of SO 2 and H 2 SO 4 has been minimized by chemical and physical processes that remove inorganic sulfur from coal (desulfurization), and by the use of coals with low sulfur content. One positive effect of higher H 2 SO 4 levels in the atmosphere is the increase in cloud cover, due to the hygroscopic (water-absorbing) nature of this acid, and this may help to lower the average surface temperature of the planet—although CO 2 produced as a result of oxidization of the carbon in coal is a major contributor to global warming.
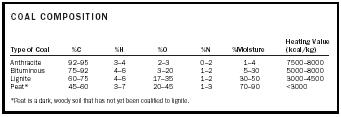
| COAL COMPOSITION | ||||||
| Type of Coal | %C | %H | %O | %N | %Moisture | Heating Value (kcal/kg) |
| *Peat is a dark, woody soil that has not yet been coalified to lignite. | ||||||
| Anthracite | 92–95 | 3–4 | 2–3 | 0–2 | 1–4 | 7500–8000 |
| Bituminous | 75–92 | 4–6 | 3–20 | 1–2 | 5–30 | 5000–8000 |
| Lignite | 60–75 | 4–6 | 17–35 | 1–2 | 30–50 | 3000–4500 |
| Peat* | 45–60 | 3–7 | 20–45 | 1–3 | 70–90 | <3000 |
Coke
Coal can be transformed into coke and other fuels by various industrial and experimental processes. Coke is produced by the pyrolysis (heating in the absence of air) of coal and is used in the production of iron and steel. The coking procedure removes moisture and other volatile components from coal, yielding an extremely carbon-rich material. Coal can also be transformed (via intrafuel conversion) into relatively clean liquid and gaseous fuels (liquifaction and gasification). However, this is accomplished at high cost—in money and energy.
Petroleum
Petroleum is an extremely complex mixture of hydrocarbons, which can be separated into liquid (oil) and gas fractions. Compared to coal, petroleum being a liquid is easier to transport. It probably originated in marine sediments, in contrast to the terrestrial origins of coal.
Because petroleum varies greatly in composition and distribution throughout the world, elaborate systems of refining and transport have been developed. Major oil fields or giant petroleum fields ("giant" indicating oil fields capable of producing at least 500 million barrels of oil) are found primarily in the Middle East, North and South America, and countries that made up the former Soviet Union. The uneven natural distribution of oil, and the consequent need to transport oil across vast distances, has led to instances of contamination due to oil spills. Coastal waters are particularly vulnerable, not only to oil spills, but also to contamination by bilge water and tank-washing water from commercial oil tankers. Even though it is a major producer of oil, the United States has found it necessary to import significant additional amounts of oil in order to meet ever-increasing industrial and home-related energy demands. Most plastics and other petrochemicals are made from petroleum, along with almost all gasoline, diesel fuel, jet fuel, heating oil, and lubricants. However, Earth's supply of petroleum is limited. Some experts estimate that world production of oil could climax as early as 2004. Although most, if not all, of the major oil-producing fields associated with continental masses have been discovered, and many offshore wells have been drilled, there still may be other major oil discoveries in less accessible areas such as under the ocean—a largely unexplored territory.
Natural Gas
The history of natural gas dates back to 900 B.C.E. , when its use was mentioned in China. It was apparently unknown in Europe until 1659, when it was discovered in England. It was not discovered in the United States until 1815 in West Virginia. In the early twenty-first century, natural gas has become the favorite fuel of industrial nations. The United States is the largest producer as well as the largest consumer of natural gas. The largest natural gas reserves are located in Russia, Kazakhstan, and Iran.
Natural gas, which consists mainly of methane (CH 4 ), can contain up to 20 percent of other gases—mainly ethane (C 2 H 6 ), and possibly propane (C 3 H 8 ), butane (C 4 H 10 ), pentane (C 5 H 12 ), carbon dioxide (CO 2 ), and nitrogen (N 2 ). Some natural gases contain small amounts of hydrogen, argon, carbon monoxide, or even hydrogen sulfide. Certain gas wells in Oklahoma also contain helium. In fact, they are a major source of helium in the United States. Natural gas is also colorless, odorless, and nontoxic but very flammable. (The odor we associate with natural gas is because of a mercaptan added to make gas leaks detectable.) Most natural gas is burned as fuel; however, ethane and the higher alkanes can be separated out and cracked to ethylene and propylene for making plastics. Although it is considered a "clean" and environmentally friendly fuel, compared to oil and coal, it is itself a major greenhouse gas and upon combustion yields carbon dioxide, the other major greenhouse gas. Like carbon dioxide, methane is also a greenhouse gas. However, natural gas fuel is thought to be only a minor contributor to methane in the atmosphere. Methane is constantly being generated by marsh and swamp terrain and by certain animals. Some experts believe that animals are the main source of atmospheric methane.
Other Sources of Fossil Fuels
Oil shales and tar sands also contain significant amounts of hydrocarbon materials that might eventually prove to be important energy sources. Oil shales are fine-grained sedimentary rocks (shales) that contain hydrocarbons that are dispersed within the matrix of the rock. A ton of shale contains from 10 to 100 gallons of kerogen, a waxy material that breaks down to oils when heated in the absence of air. It is estimated that three states (Utah, Colorado, and Wyoming) contain shale bearing more oil than exists in all the proven reserves in the world. Tar sands are the extremely viscous petroleum deposits associated with sedimentary rocks. They are mixtures of clay, sand, and extremely viscous oils called bitumens. The utility of oil shales and tar sands is currently limited, because of problems having to do with hydrocarbon recovery and the disposal of large amounts of inorganic residues.
SEE ALSO Chemistry and Energy ; Coal ; Energy Sources and Production ; Gasoline ; Industrial Chemistry, Organic ; Petroleum .
Mary L. Sohn
Bibliography
Spiro, Thomas G., and Stigliani, William M. (1996). Chemistry of the Environment. Upper Saddle River, NJ: Prentice-Hall.
Yeh, The Fu (1999). Environmental Chemistry: Essentials of Chemistry for Engineering Practice, Vol. 4A. Upper Saddle River, NJ: Prentice-Hall.