Gasoline

In 1859 Edwin Drake and E. B. Bowditch of the Seneca Oil Company drilled the first commercial oil well in the United States in Titusville, Pennsylvania. The well produced about 400 gallons of crude oil, less than ten barrels a day. Soon, similar wells all over western Pennsylvania were providing crude oil for kerosene production that was needed to fuel the nation's streetlights and house lamps. The lighter boiling component, gasoline, was discarded, since it had no market. There are historical reports that "waste" gasoline, which had been dumped into rivers, sometimes caught fire. In 1892 the first gasoline-powered engines, for both car and tractor, were developed: This soon provided a market for the once useless substance, gasoline.
Today gasoline is the most important product of a typical oil refinery: The entire refinery process is designed to maximize its production. Gasoline is a complex mixture of molecules with a boiling range of 40–200°C (104–392°F). To produce various grades, there is a blending of many refinery components, each of which promotes specific fuel qualities such as desired octane rating, volatility, and minimization of engine deposits.
Octane Quality
The most important quality parameter for gasoline is the octane quality. Octane number is a measure of the antiknock properties of the fuel. Knocking in a gasoline engine is a metallic clattering noise (pinging), which indicates excessive intensity in preflame reactions. Severe knocking can damage the engine.
Preflame reactions occur in the engine cylinders when portions of the fuel self-initiate combustion prior to the advancing flame from the spark plug. This additional combustion causes an excessive rate of energy release, which is knock. The tendency of a fuel to engage in preflame reactions is dependent upon the structure of its component molecules (see Figure 1);
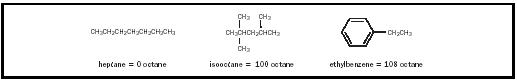
the tendency for preflame reactions is high for straight chain hydrocarbons, medium for branched hydrocarbons, and low for aromatics.
The octane number for a test gasoline represents the percentage by volume of isooctane (2,2,4-trimethylpentane) in a reference fuel consisting of the mixture of isooctane and heptane that would be necessary to match the test fuel's knocking tendency. Isooctane burns with a minimal knocking and is given an octane rating of 100. This is in contrast to heptane, which burns with much knocking and is given an octane rating of 0. Thus, a gasoline that burns with the same amount of knocking as a mixture of 92 percent isooctane and 8 percent heptane is classified as a 92 octane gasoline.
The octane ratings of gasoline can be increased by the addition of small amounts of antiknock agents. The first commercially successful antiknock agent, tetraethyllead (TEL), was developed in the 1920s. TEL was used to promote the development of higher efficiency, higher compression engines. However, TEL is highly toxic and poisons catalytic converters. Since 1974 all new U.S. automobile engines have used catalytic converters in order to reduce exhaust emissions.
Methyl t -butyl ether (MTBE) has been the antiknock agent of choice for unleaded gasoline. MTBE provides high-octane quality along with low volatility and is readily soluble in gasoline. However, leakage of gasoline from underground storage tanks has resulted in the detection of MTBE in the drinking water of several urban areas. This prompted the state of California to order the removal of MTBE from California gasoline by 2003.
Alcohols also have found use as octane enhancers. At higher concentration alcohols can be used as gasoline extenders, thus decreasing our dependency upon imported crude oil. A significant portion of all U.S. marketed gasoline is believed to contain ethanol.
Gasoline Additives
Trace amounts of olefins and diolefins found in gasoline are prone to reaction with oxygen dissolved in the gasoline. This process is referred to as autoxidation and involves a radical chain reaction that can incorporate oxygen
into the olefin and also can promote a molecular size increase via polymerization reactions. The end result of this complex process is the formation of deposits and gums that can block fuel filters and interfere with the metering of fuel and air in the carburetor. This can result in adverse engine performance. Additives are frequently added to gasoline to address oxidative stability and other issues; they include antioxidants, metal deactivators, and detergents.
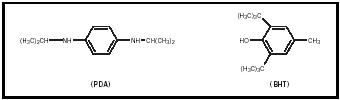
Antioxidants are additives that minimize autoxidation reactions. They function as hydrogen atom donors that stop the chain oxidation process of the olefins. The two different types of antioxidants used in gasoline are phenylenediamines (PDA) and hindered phenols (such as BHT).
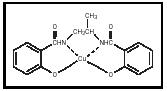
Trace levels of soluble metal compounds, particularly copper, catalyze the oxidative degradation of gasoline by promoting the formation of gums and deposits. Metal deactivators overcome this problem by chelating the metal and rendering it inactive. The most widely used metal deactivator is N, N′-disalicylidene-1,2-propanediamine, the copper complex of which is shown in Figure 3.
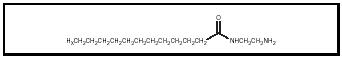
Detergents minimize fuel system deposits at low concentrations, and at high concentrations can remove deposits that have already formed. Detergents are molecules that have a highly polar end group and a nonpolar hydrocarbon tail. A conventional amino amide type detergent is shown in Figure 4.
Presumably, the polar groups in the detergent attach themselves to metal surfaces and to polar deposits on these surfaces. The nonpolar tails of these molecules "stick out" into the fuel in such a way that a monomolecular film is formed on the metal surface, preventing deposition and particle aggregation. This process is also believed to solubilize any deposits already on the metal surface. The detergent monolayer is also believed to prevent the buildup of ice on carburetor surfaces during winter. Thus, detergents can also function as anti-icing additives.
Gasoline Production
The production of gasoline begins with desalting the viscous crude oil. Salts and metals in the crude oil promote corrosion and poison processing catalysts. Thus, the crude oil is heated (to decrease viscosity) and extracted with water to remove the salts and metals. Frequently this process results in the formation of an oil/water mixture referred to as an emulsion (suspension). This emulsion is typically broken by the addition of a chemical surfactant (demulsifier) that promotes the separation of discrete oil and water layers. After separation of the aqueous layer, the oil is heated to about 400 o C (752 o F): This converts the oil into gaseous products and increases the fluidity of the remaining liquid. In this form, the gaseous mixture enters the fractionating column, where the process of atmospheric fractional distillation separates the crude oil into different components based upon boiling point.
The lightest boiling fractions are molecules that are gases under ambient conditions: methane, ethane, propane, butane, and olefins derived from these compounds. Uses for this distillate stream include burning as a fuel at the refinery; as petrochemical feed stocks; or processing into liquefied petroleum gas (LPG). There are three other major distillate streams collected during atmospheric distillation: the naptha fraction, which has a boiling range of 30 to 180°C (86–356°F); the kerosene fraction, which distills at between 180 and 240°C (356–464°F); and the gas oil fraction, which distills at between 240 and 355°C (464–671°F).
In order to meet current environmental regulations for the sulfur content in fuel products, the three-distillate streams are subjected to the process of hydrodesulfurization. In the presence of a catalyst , distillates are heated in the presence of hydrogen to reduce various organosulfur compounds to simple organic compounds and H 2 S. The hydrogen needed for this process is a by-product of the catalytic reforming process. The H 2 S product can be readily removed. In this process the refiner can control the octane number of the gasoline blending stock. By heating the naphtha fraction in the presence of an especially designed platinum catalyst, straight-chain hydrocarbons are cyclized, and saturated cyclic hydrocarbons are converted into aromatic compounds. In addition, this process converts straight-chain hydrocarbons into branched hydrocarbons. Catalytic reforming facilitates the production of gasoline blending stocks with octane ratings of from 90 to 100+.
Redistilling the atmospheric residue at a temperature of less than 400°C (752°F) under vacuum produces a vacuum gas oil. Typically, the vacuum gas oil is subjected to fluid catalytic cracking (FCC) to produce lower-boiling liquids that can be blended to make gasoline. This is achieved by breaking large molecules of the vacuum gas oil into smaller, lower-boiling molecules. An important gasoline blending component that can be produced in this manner is alkylate. It is a mixture of highly branched hydrocarbons produced by the acid-catalyzed reaction of isobutene and light olefinic hydrocarbons. Alkylate is a valuable blending component because of its high-octane quality and the absence of aromatics or olefins, which can lead to environmental and oxidative stability problems.
The 1990 Clean Air Act required the Environmental Protection Agency (EPA) to issue regulations that required gasoline to be "reformulated," resulting in significant reductions in vehicle emissions of ozone-forming and toxic air pollutants. This cleaner gasoline is called reformulated gasoline (RFG). RFG is required in the nine major metropolitan areas in the United States having the worst ozone problems. In addition, several other areas with ozone levels exceeding the public health standard have voluntarily chosen to use RFG.
Use of RFG decreases the amounts of volatile organic compounds (VOCs) and oxides of nitrogen (NO x ) in the atmosphere that react in the presence of sunlight to produce ozone, a major component of smog. Vehicles also release toxic emissions, one of which (benzene) is a known carcinogen.
RFG contains 2 percent by weight oxygen additives (oxygenates), such as MTBE or ethanol. Oxygenates increase the combustion efficiency of gasoline, reducing vehicle emissions of carbon monoxide, a serious public health threat. The appearance of MTBE in some urban water supplies has resulted in legislation pending in the U.S. Congress to phase out the use of MTBE in RFG. Ethanol would then most likely become the primary oxygenate for future RFG.
Gasoline is the most important product of the oil refinery. The most important quality parameter for gasoline is its octane number. Additional quality characteristics for gasoline are controlled by the use of additives, for example, antioxidants, metal deactivators, and detergents. By blending various refinery streams and additives a gasoline formulation can be achieved that minimizes environmental degradation. Such a fuel is called reformulated gasoline.
SEE ALSO Detergents ; Energy Sources and Production ; Fossil Fuels ; Petroleum .
Bruce Beaver
Bibliography
Marshall, E. L., and Owen, K., eds. (1995). Critical Reports on Applied Chemistry, Vol. 34: Motor Gasoline. Cambridge, U.K.: Royal Society of Chemistry.
Comment about this article, ask questions, or add new information about this topic: