Petroleum

Petroleum is a naturally occurring complex mixture made up predominantly of carbon and hydrogen compounds, but also frequently containing significant amounts of nitrogen, sulfur, and oxygen together with smaller amounts of nickel, vanadium, and other elements. Solid petroleum is called asphalt; liquid, crude oil; and gas, natural gas. Its source is biological. Organic matter buried in an oxygen-deficient environment and subject to elevated temperature and pressure for millions of years generates petroleum as an intermediate in the transformation that ultimately leads to methane and graphite. The first successful drilled oil well came in 1859 in Pennsylvania. This is considered to be the beginning of the modern oil industry. Continuous distillation of crude oil began in Russia in 1875.
Occurrence
Oil is the largest segment of our energy raw materials use, being 40 percent, while coal use accounts for 27 percent, gas 21 percent, and hydroelectric/nuclear 12 percent. Although there are 20,000 petroleum fields known worldwide, more than half of the known reserves are contained in the 51 largest fields. The Middle East has 66 percent of the known world reserves. The United States has only 2 percent of the known world reserves. Hence the need for imports. The Organization of Petroleum Exporting Countries (OPEC) is important to the international trade and distribution of this crude oil. There is a growing dependence of the United States on imports. Although U.S. domestic production has not grown since the 1950s, imports have grown dramatically, from 0.3 billion barrels of oil in 1955 to 3.0 billion barrels in 1997. The United States has increased its percentage of imports, from approximately 13 percent in 1970 to 55 percent in 2000. It uses approximately 18 million barrels of oil per day. Worldwide production is about 56 million barrels per day. With known reserves, this level of worldwide production could remain constant for only 43 years. But there are large volumes of unconventional petroleum reserves, such as heavy oil, tar sands, and oil shale. These are located in the Western Hemisphere. Improvements in recovery methods must be made, and the cost of production must decrease, for these sources to become more important providers of energy.

Composition
Crude oils vary dramatically in color, odor, and flow properties. There are light and heavy crude oils; they are sweet or sour (i.e., have high or low sulfur content, with an average of 0.65%). Several thousand compounds are present in petroleum. The number of carbon atoms in these compounds can vary from one to over a hundred. Few are separated as pure substances. Many of the demands for petroleum can be served by certain fractions obtained from the distillation of crude oil. Typical distillation fractions and their uses are given in Table 1. The complexity of the molecules, their molecular weights, and their carbon numbers increase with the boiling point. The higher-boiling fractions are usually distilled in vacuo at temperatures lower than their atmospheric boiling points to avoid excessive decomposition to tars.
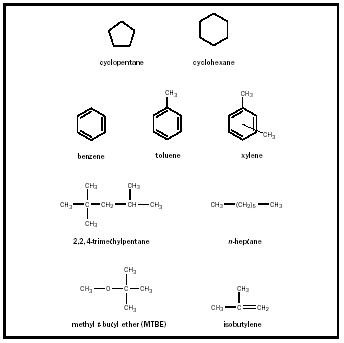
Each fraction of distilled petroleum is a complex mixture of chemicals, but these mixtures can be somewhat categorized. A certain sample of straight-run gasoline (light naphtha) might contain nearly 30 aliphatic (containing no benzene ring), noncyclic hydrocarbons; nearly 20 cycloaliphatic hydrocarbons (mainly cyclopentanes and cyclohexanes), sometimes called
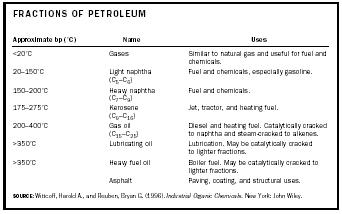
| FRACTIONS OF PETROLEUM | ||
| Approximate bp (°C) | Name | Uses |
| SOURCE : Wittcoff, Harold A., and Reuben, Bryan G. (1996). Industrial Organic Chemicals . New York: John Wiley. | ||
| <20°C | Gases | Similar to natural gas and useful for fuel and chemicals. |
| 20–150°C | Light naphtha (C 5 –C 6 ) | Fuel and chemicals, especially gasoline. |
| 150–200°C | Heavy naphtha (C 7 –C 9 ) | Fuel and chemicals. |
| 175–275°C | Kerosene (C 9 –C 16 ) | Jet, tractor, and heating fuel. |
| 200–400°C | Gas oil (C 15 –C 25 ) | Diesel and heating fuel. Catalytically cracked to naphtha and steam-cracked to alkenes. |
| >350°C | Lubricating oil | Lubrication. May be catalytically cracked to lighter fractions. |
| >350°C | Heavy fuel oil | Boiler fuel. May be catalytically cracked to lighter fractions. |
| Asphalt | Paving, coating, and structural uses. | |
naphthenes; and 20 aromatic compounds (such as benzene, toluene, and xylene). Examples of compounds found or used in petroleum and mentioned in this article are given in Figure 1.
When any fraction of petroleum is used as a source of energy and burned to CO 2 and H 2 O, the sulfur is converted into SO 2 in the air. The SO 2 is a major air contaminant, especially in larger cities. With air moisture it can form H 2 SO 4 and H 2 SO 3 . Much of the sulfur-containing material must be taken out of petroleum before it can be used as fuel. The current maximum percentage allowable in gasoline is 0.10 percent S.
Octane Number
One cannot talk about the chemistry of gasoline without understanding octane numbers. When gasoline is burned in an internal combustion engine to CO 2 and H 2 O, there is a tendency for many gasoline mixtures to burn unevenly. Such nonconstant and unsmooth combustion creates a "knocking" noise in the engine. Knocking signifies that the engine is not running as efficiently as it could. It has been found that certain hydrocarbons burn more smoothly than others in a gasoline mixture. In 1927 a scale that attempted to define the "antiknock" properties of gasolines was created. At that time, 2,2,4-trimethylpentane (commonly called "isooctane") was the hydrocarbon that, when burned pure in an engine, gave the best antiknock properties (caused the least knocking). This compound was assigned the number 100, meaning it was the best hydrocarbon to use. The worst hydrocarbon researchers could find in gasoline (which when burned pure gave the most knocking) was n -heptane, assigned the number 0. When isooctane and heptane were mixed, they gave different amounts of knocking depending on their ratio: The higher the percentage of isooctane in the mixture, the lower was the amount of knocking. Gasoline mixtures obtained from petroleum were burned for comparison. If a certain gasoline has the same amount of knocking as a 90 percent isooctane, 10 percent heptane (by volume) mixture, we now say that its "octane number" is 90. Hence, the octane number of a gasoline is the percent isooctane in an isooctane-heptane
mixture that gives the same amount of knocking as the gasoline being measured. Thus, a high octane number means a low amount of knocking.
Presently there are two octane scales, a research octane number (RON) and a motor octane number (MON). RON values reflect performance at 600 rpm, 148.8°C (125°F), and low speed. MON is a performance index of driving with 900 rpm, 51°C (300°F), and high speed. The station pumps now give the (R + M)/2 value. Regular is usually 87 to 89 and premium about 92 on this scale.
Certain rules have been developed for predicting the octane number of different types of gasoline, depending on the ratio of different types of hydrocarbons in the mixtures:
- The octane number increases as the amount of branching or the number of rings increases.
- The octane number increases as the number of double and triple bonds increases.
Additives
In 1922 two chemists working at General Motors, Midgley and Boyd, were looking at different substances that would aid the combustion of gasoline and help the knocking problems of engines. In other words, they were seeking methods of increasing the octane rating of gasoline without altering the

hydrocarbon makeup. They were also interested in cleaning up the exhaust of automobiles by eliminating pollutants such as unburned hydrocarbons and carbon monoxide through more complete combustion. By far the best substance that they found was tetraethyllead. Lead in this form aids in breaking carbon-carbon and carbon- hydrogen bonds . But the lead oxide formed in the combustion is not volatile and would accumulate in the engine if dibromoethane and dichloroethane were not added. In the environment the lead dihalide formed undergoes reaction by sunlight to elemental lead and halogen , both of which are serious pollutants.
For the past several years other additives have been tried. Ethyl alcohol has become popular. When 10 percent ethyl alcohol is mixed with gasoline it is called gasohol and it is popular in states with good corn crops, as the alcohol can be made from corn fermentation. An attractive alternative to tetraethyllead is now methyl t -butyl ether (MTBE). MTBE has been approved at the 7 percent level since 1979. From 1984 to 1995 its production grew by 25 percent per year, the largest increase of any of the top chemicals. The Clean Air Act of 1991 specifies that the gasoline must be at the 2.0 percent oxygen level. Thus, MTBE, ethyl t -butyl ether (ETBE), ethanol, methanol, and other ethers and alcohols had to be added to gasoline at higher levels. The product is called reformulated gasoline (RFG), and it may cut carbon monoxide levels and may help to alleviate ozone depletion. But improved
emission control systems may make this high-level input unnecessary. Currently MTBE accounts for 85 percent of the additive market, with 7 percent being ethanol and the remaining 8 percent split by other chemicals. In 1999 California took steps toward banning MTBE. In 2000 some factions called for a U.S. ban on MTBE and for increased use of ethanol to meet the oxygenate requirement. MTBE has been found in drinking water. But ethanol cannot be blended into gasoline at the refinery because it is hygroscopic and picks up traces of water in pipelines and storage tanks. Also, ethanol shipped away from the Midwest, where it is made by corn fermentation, would add to the cost of gasoline. Gasohol may increase air pollution because gasoline containing ethanol evaporates more quickly. Studies and debate continue.
Refinery Processes
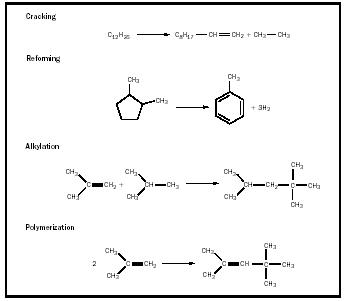
There are processes that are used to refine petroleum into useful products. These are important processes for the gasoline fraction because they increase the octane rating. Some of these processes are used to increase the percentage of crude oil that can be used for gasoline. They were developed in the 1930s when the need for gasoline became great with the growing automobile industry. These processes are also keys in the production of organic chemicals. An example of each of these processes is given in Figure 2. One process is cracking. In catalytic cracking, as the name implies, petroleum fractions of higher molecular weight than gasoline can be heated with a catalyst and cracked into smaller molecules. This material can then be blended into the refinery gasoline feed.
Catalytic reforming leaves the number of carbon atoms in the feedstock molecules usually unchanged, but the resultant mixture contains a higher number of double bonds and aromatic rings. Reforming has become the principal process for upgrading gasoline. High temperatures with typical catalysts of platinum and/or rhenium on alumina and short contact times are used. A typical example is the reforming of dimethylcyclopentane to toluene. Straight-run gasoline can be reformed to as high as 40 to 50 percent aromatic hydrocarbons, of which 15 to 20 percent is toluene.
Although cracking and reforming are by far the most important refinery processes, especially for the production of petrochemicals, two other processes deserve mention. In alkylation, alkanes (hydrocarbons with no double or triple bonds) react with alkenes (hydrocarbons with double bonds) in the presence of an acid catalyst to give highly branched alkanes. In polymerization an alkene can react with another alkene to generate dimers, trimers, and tetramers of the alkene. As an example, isobutylene (C 4 ) reacts to give a highly branched C 8 alkene dimer.
Natural Gas
Natural gas can be as high as 97 percent methane, the remainder being hydrogen, ethane, propane, butane, nitrogen, hydrogen sulfide, and heavier hydrocarbons. A typical mixture contains 85 percent methane, 9 percent ethane, 3 percent propane, 1 percent butanes, and 1 percent nitrogen. Uses of natural gas by all industry include fuel (72%) and the manufacture of: inorganic chemicals including ammonia (15%), organic chemicals (12%), and carbon black (1%). The ethane and propane are converted to ethylene and propylene. The methane is purified and used to make a number of other chemicals.
SEE ALSO Energy Sources and Production ; Fire, Fuels, Power Plants ; Fossil Fuels ; Gasoline ; Industrial Chemistry, Organic .
Philip J. Chenier
Bibliography
Chenier, Philip J. (2002). Survey of Industrial Chemistry, 3rd edition. New York: Kluwer Academic/Plenum Publishers.
Wittcoff, Harold A., and Reuben, Bryan G. (1996). Industrial Organic Chemicals. New York: Wiley.