Transmutation

Transmutation is the act of changing a substance, tangible or intangible, from one form or state into another. To the alchemists of old, this meant the conversion of one physical substance into another, particularly base metals such as lead into valuable silver and gold. To the modern scientists, this means the transformation of one element into another by one or a series of nuclear decays or reactions.
Although people worked with gold, silver, copper, iron, tin, lead, carbon, sulfur, and mercury in ancient times, they had little understanding of chemistry and could write little about it. At this time chemistry was an art, not a science. The Egyptians were the first to produce extensive written documentation of chemical procedures, at the beginning of the Christian era, and Egypt is generally identified as the birthplace of chemistry. These writings indicate that the development of methods for transmuting one substance into another was one of the principal early goals of their investigations. During the several hundred years that followed these writings, the alchemists attempted to develop schemes to transmute base metals into gold and silver through various chemical manipulations of mixtures and distillations. The alchemists were spurred on by what appeared to be some success—for example, production of very small amounts of gold from lead ore by their chemical procedures. (This gold was undoubtedly present in trace amounts in the original ores and was not produced by transmutation.) The ultimate folly of the alchemists turned up during the Middle Ages with the search for the philosopher's stone, a substance that could be mixed with base metals and, through purification, convert them into gold. It was never found.
Because each element has a different but fixed number of protons in the nucleus of the atom, which is the atomic number, the transmutation of one chemical element into another involves changing that number. Such a nuclear reaction requires millions of times more energy than was available through chemical reactions. Thus, the alchemist's dream of transmuting lead into gold was never chemically achievable.
Although the alchemists failed to find a method for the transmutation of base metals into precious metals, a number of important chemical processes resulted from their efforts. For example, they extracted metals from ores; produced a number of inorganic acids and bases that later became commercially important; and developed the techniques of fusion, calcination, solution, filtration, crystallization, sublimation, and, most importantly, distillation. During the Middle Ages, they began to try to systematize the results of their primitive experiments and their fragments of information in order to explain or predict chemical reactions between substances. Thus the idea of chemical elements and the first primitive forms of the chemical Periodic Table appeared.
Ironically, nuclear transmutations were taking place virtually under the noses of the alchemists (or under their feet), but they had neither the methods to detect nor the knowledge to use these happenings. The discovery of the nuclear transmutation process was closely linked to the discovery of radioactivity by Henri Becquerel in 1896. Nuclear transmutations occur during the spontaneous radioactive decay of naturally occurring thorium and uranium (atomic numbers 90 and 92, respectively) and the radioactive

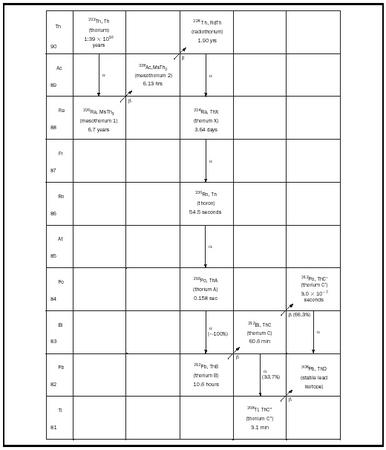
daughter products of their decay, namely the natural decay series. As an example, the 232 Th decay series is shown in Figure 1. The superscript 232 represents the atomic mass, which is the total number of protons and neutrons in the nucleus of the atom. In this decay series, a 232 Th nucleus starts the process by spontaneously emitting an α -particle (a He nucleus containing two protons and two neutrons). This reaction transmutes the Th nucleus into a nucleus with two fewer protons and two fewer neutrons, namely 228 Ra. Then the 228 Ra nucleus spontaneously emits a β -particle (an electron), which converts a neutron in the nucleus into a proton, raising the atomic number of the resultant nucleus by one with no change in atomic mass, yielding 228 Ac. This sequence of successive α and β decays continues from one element to another until the stable 208 Pb nucleus is produced (see Figure 1). There exist two other naturally occurring decay chains as well, one starting with 235 U (the actinium series) and one starting with 238 U (the uranium series). In addition to these three decay series, fourteen other radioactive isotopes exist in nature, ranging from 40 K to 190 Pt, which transmute by decay into stable elements.
The idea of transmutation of elements in the natural decay chains did not accompany the discovery of radioactivity by Becquerel. However, Marie and Pierre Curie extended the investigations of Becquerel using a variety of
U minerals and found the radioactive properties to be not a function of the physical or chemical forms of the uranium, but properties of the element itself. Using chemical separation methods, they isolated two new radioactive substances associated with the U minerals in 1898 and named them polonium and radium. In 1902 Ernest Rutherford and Frederick Soddy explained the nature of the process occurring in the natural decay chains as the radioactive decays of U and Th to produce new substances by transmutation.
Lord Rutherford and his group of scientists were the first persons to produce and detect artificial nuclear transmutations in 1919. He bombarded nitrogen in the air with the α -particles emitted in the decay of 214 Po. The transmutation reaction involved the absorption of an α -particle by the 14 N nuclei to produce 17 O and a proton (a hydrogen nucleus). This reaction can be written as
14 N + 4 He → 17 O + 1 H
Lord Rutherford was able to detect and identify the protons produced in this nuclear reaction and thereby demonstrate the transmutation process.
Until 1934, only naturally occurring radioactive elements were available for study. However, in January of that year, Irene Curie (daughter of Marie Curie) and Frederic Joliot reported that boron and aluminum samples were made radioactive by bombarding them with α -particles from polonium to produce the two new radioactive products, 13 N and 30 P respectively. This discovery established the new fields of nuclear chemistry and radiochemistry and sparked their rapid growth.
With the development of nuclear reactors and charged particle accelerators (commonly referred to as "atom smashers") over the second half of the twentieth century, the transmutation of one element into another has become commonplace. In fact some two dozen synthetic elements with atomic numbers higher than naturally occurring uranium have been produced by nuclear transmutation reactions. Thus, in principle, it is possible to achieve the alchemist's dream of transmuting lead into gold, but the cost of production via nuclear transmutation reactions would far exceed the value of the gold.
SEE ALSO Alchemy ; Nuclear Chemistry ; Nuclear Fission ; Radioactivity ; Transactinides .
Robert J. Silva
Bibliography
Armitage, F. P. (1906). A History of Chemistry. New York: Longmans, Green.
Freidlander, Gerhart; Kennedy, Joseph W.; and Miller, Julian Malcolm (1964). Nuclear and Radiochemistry, 2nd edition. New York: Wiley.
Partington, J. R. (1957). A Short History of Chemistry, 3rd edition. New York: Macmillan.
Romer, Alfred, ed. (1964). The Discovery of Radioactivity and Transmutation. New York: Dover Publications.
Seaborg, Glenn T., and Valens, Evans G. (1958). Elements of the Universe. New York: Dutton.
Seaborg, Glenn T. (1963). Man-Made Transuranium Elements. Englewood Cliffs, NJ: Prentice-Hall.