Molecular Structure

The Rise and Reemergence of Atomism
Throughout history, humans have created models to help them explain the observed character of substances and phenomena in the material world. The ancient philosophers Democritus and Lucretius were among the first to speculate that matter was discontinuous, and that small, indivisible particles not only made up substances but also gave them their observed properties. The Greeks called these particles "atoms" (the English equivalent), a word that meant indivisible. Lucretius imagined that the particles that made up vapor had smooth surfaces and could not interconnect, giving vapors (gases) their extreme mobility. Liquids, on the other hand, were thought to be made up of particles, each particle having a few hooks. These few hooks would get entwined but would not immobilize the particles, thereby causing the particles to cling, yet still be fluid. The particles that made up solids, by contrast, were thought to have many hooks, resulting in the extremely sturdy nature of solid materials. The hypothesis of finite particles implied empty space between them. Yet, the majority of Greek philosophers did not believe that nothingness (the vacuums between particles) could exist, so the idea of atoms did not last long in the ancient times. Ironically, the objection was not to the existence of particles, but to the vacancies that must exist between them.
Most cultures have linked properties of matter with religious and/or superstitious ideas. The term "gold" derives from an Old English word meaning "something shiny and yellow like the Sun"; it served not only as the name of the metal but also identified its properties. Polished gold nearly captures the sunlight it reflects, and the astronomical, astrological, medical, and religious attributes of the Sun were thought to be present in gold metal. For thousands of years, substances were said to contain essences or essential parts that gave them their characters. In a sense modern ideas about molecular structure do something similar. Chemists construct explanations for observed, macroscopic phenomena (e.g., reactivity) by describing the assemblages, shapes, and motions of submicroscopic particles.
The theory of atoms did not reemerge until the seventeenth century. The discovery of elements rapidly led to the idea that nonelementary substances were made up of molecules that were, in turn, collections of elemental atoms. During the first years of chemical analyses, different substances were observed to have different compositions; the deduction was made that substances were different because their compositions were different. One type of mineral might be 34 percent iron and 66 percent oxygen. Each sample of that mineral would give the same results (34% iron and 66% oxygen). A different mineral, that is, one with different properties, might be 56 percent iron and 44 percent oxygen. Although there was still no concept of bonding between atoms or of molecular geometry at the beginning of the nineteenth century, chemists had developed the idea that different molecules were different collections of atoms.
Isomerism and the Development of Molecular Structural Models
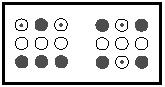
Scientific theories are sometimes discarded. When information that contradicts a theory is reliable, the theory must be changed to fit the new data. As the elemental analysis of compounds expanded greatly during the early 1800s, observations that different substances were of the same elemental composition were inevitable. In his History of Chemistry (1830), Thomas Thomson drew illustrations of varying hypothetical particle arrangements, using symbols that were used at that time (those of John Dalton), as a way to explain why two acids of the same elemental composition could have different physical and chemical properties (see Figure 1). These are believed to be the earliest recorded representations of molecular structure that showed varying arrangements of the same atoms; the phenomenon would soon be called isomerism (from the Greek iso, meaning same, and meros, meaning part). In 1828 Friedrich Wöhler (1800–1882) synthesized urea, (NH 2 ) 2 C = O or CH 4 N 2 O, that was indistinguishable from that that had been isolated from urine. He prepared this organic substance from the clearly inorganic (mineralogical) starting material ammonium cyanate, NH 4 (+) NCO(−), also CH 4 N 2 O, the result of the combination of ammonium chloride and silver cyanate. Urea and ammonium cyanate are constitutional isomers , and together illustrate the fact that fixed arrangements of atoms, molecular structures, must be invoked to explain observed phenomena.
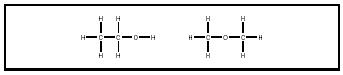
The constitution of a molecule (number of, kind of, and connectivities of atoms) may be represented by a two-dimensional "map" in which the interatomic linkages (bonds) are drawn as lines. There are two constitutional isomers that are represented by the molecular formula C 2 H 6 O: ethanol and dimethyl ether. The differences in connectivities, which are not evident in the common constitutional inventory C 2 H 6 O, can be conveyed by typographical line formulas (CH 3 CH 2 OH for ethanol and CH 3 OCH 3 for dimethyl ether), or by structural representations (see Figure 2). As the number and kinds of atoms in substances increase, the number of constitutional isomers increases.
By the mid-1850s, a new theory of molecular structure had emerged. Given a unique collection of atoms, it was not the identities of the atoms that distinguished one molecule from another, but rather the connectivity,
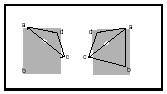
or bonding, of those atoms. The nature of the chemical bond was unknown, and the phenomenon of chemical bonding was described as "chemical affinity." Because it was observed that the passing of electricity through some substances, such as water, could "break" the molecules apart into their elements (electrolysis), the electrostatic attractions of charged particles (ions) were used to contribute to an explanation of chemical affinity. Just as the hypothesis of the varying connectivities of atoms emerged as a response to observations that could not be explained, variation in the three-dimensional arrangements of atoms in space was proposed to reconcile other observed phenomena. Jacobus van't Hoff (1852–1911) and Joseph-Achille Le Bel (1847–1930) proposed (independently of one another, in 1874) that molecules of the same connectivity yet different physical properties (e.g., optical activity) might be explained if, in the case of four different particles, the arrangement (configuration) of the particles was tetrahedral. Macroscopically or microscopically, a tetrahedral array of four different things gives rise to two and only two different arrangements that are nonsuperimposable mirror images (enantiomers; see Figure 3). Distinct molecular structural units that have the same connectivities but varying three-dimensional arrangements are also isomers. The term "stereoisomer" was introduced by Viktor Meyer in 1888 to describe molecules that differ only in their three-dimensional arrangements.
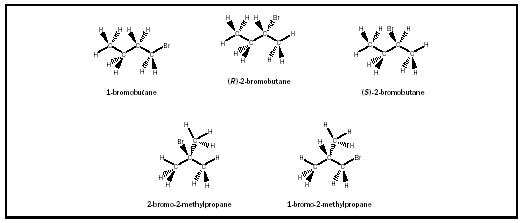
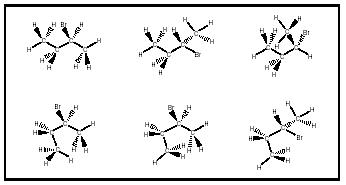
Connectivity and stereoisomerism give chemists a way to uniquely differentiate one molecular structure from another. The molecular formula C 4 H 9 Br, for instance, represents five different substances (see Figure 4). Predictably, although there is only one compound for each of the connectivities designated 1-bromobutane, 2-bromo-2-methylpropane, and 1-bromo-2-methylpropane, there are two compounds represented by the connectivity designated 2-bromobutane (carbon 2 has four different groups attached, and thus two three-dimensional arrangements of the molecule, whose geometries are labeled R and S, exist). There are no other isomers of C 4 H 9 Br that are predicted, and none that are observed.
Although the arrangement of molecular atoms around a given point is fixed, molecules are not static objects. The sequence of links in a chain, for instance, is constant, but the chain can be twisted and knotted into countless shapes. In the case of a molecule, twists do not affect the identity of a substance, but the overall molecular shape is part of molecular structure and can have an impact on the observed properties. According to Ernest Eliel and Samuel Wilen (1994, p. 102), configurational stereoisomers result from "arrangements of atoms in space of a molecule with a defined constitution, without regard to arrangements that differ only by rotation about one or more single bonds, providing that such a rotation is so fast as not to allow isolation of the species so differing." Conformational stereoisomers are
molecular shapes resulting from bond rotations that do not affect molecular identity . The drawings shown in Figure 5 represent some of the different conformational shapes that the single molecule ( S )-2-bromobutane can assume.
The overall geometry of a molecule was recognized as contributing to its chemical reactivity in the 1950s, and methods used to determine molecular structure have grown dramatically since that time. Throughout the early 1900s, direct experimental evidence of the three-dimensional arrangements of atoms was becoming available as a result of x-ray diffraction crystallography. Nuclear magnetic resonance spectroscopy (first used in the 1960s) and atomic force microscopy (in the 1980s) are two techniques of many that are now used to gather experiment-based information about molecular structure. What might have taken years to determine in 1950, and what was impossible to know about extremely large biopolymers (e.g., DNA , enzymes, and polysaccharides at a cell surface) as late as 1990 can now sometimes be determined in a matter of seconds.
Molecular environment influences molecular structure. The shape that a molecule assumes within a crystal lattice is necessarily different from its shape in water and will vary according to solvent and other environmental factors (e.g., temperature and pH). Beginning in the late 1980s the significance of the noncovalent aggregation of large numbers of molecular entities began to be understood. A protein, for instance, folds into its three-dimensional shape because water is present; without water, the shape is quite different. Thus, molecular structure is determined by a combination of extrinsic as well as intrinsic factors. The field of molecular structure and reactivity that deals with large aggregations of molecules and how they influence each other is called supramolecular chemistry.
Molecular Structural Theory
The electron was discovered in 1900, and it took about twenty years for the electronic nature of the chemical bond to come into wide acceptance. Particle-based models for atomic and molecular structure soon gave way to the quantum mechanical view, in which electrons are not treated as localized, discrete particles (electrons orbiting around a nucleus), but as delocalized areas of wavelike charge, each possessing a given probability of being found in a given location near an atomic nucleus (an orbital). The chemical bonding in molecules, which began the twentieth century as shared electron pairs between atoms, evolved to become a matter of molecular orbitals. Molecular orbitals describe three-dimensional arrangements of the atomic nuclei in a molecule and the probability that any given electron of a given energy will occupy a given location with respect to those nuclei. Single bonds are explained by the overlap of atomic orbitals along the internuclear axis of two atoms. Multiple bonds are the combination of sigma plus pi bonding, the latter corresponding to the overlap of atomic orbitals that is not along the internuclear axis. A rough guide to the bonding molecular orbitals in methane is depicted in Figure 6. The eight valence shell electrons (four from carbon, four from the four hydrogens) are
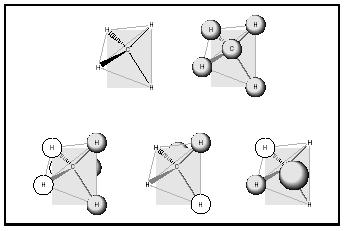
distributed among four molecular orbitals. One of the four orbitals is composed of favorable bonding interactions between the 2 s -orbital of carbon and the four 1 s -orbitals of the hydrogen atoms, whereas the other three are the equally likely combinations of one of the three 2 p -orbitals of carbon and the 1 s orbitals of hydrogen atoms. Computer-based models for chemical bonding are as important to modern molecular structural theory as experimental measurements.
SEE ALSO Isomerism ; Le Bel, Joseph-Achille ; Molecules ; Nuclear Magnetic Resonance ; VAN'T Hoff, Jacobus ; WÖhler, Friedrich .
Brian P. Coppola
Bibliography
Eliel, Ernest L., and Wilen, Samuel H. (1994). Stereochemistry of Organic Compounds. New York: Wiley.
Thomson, Thomas (1830). The History of Chemistry. London: Colburn and Bentley.
Comment about this article, ask questions, or add new information about this topic: