Molecules
A molecule is the smallest entity of a pure compound that retains its characteristic chemical properties, and consequently has constant mass and atomic composition. It is an assembly of nonmetallic atoms held together into specific shapes by covalent bonds . As much as a car is a single unit made up of many parts, a molecule is a unit made up of atoms bonded around each other in certain fixed geometries. Shapes influence the physical and chemical properties and consequently much of the chemistry of a molecule.
While molecules may be monoatomic (such as the inert gases helium, neon, or krypton), most molecules are diatomic, triatomic, or polyatomic, consisting of two or more atoms (some molecules may be a collection of thousands of atoms). A diatomic molecule may be homonuclear (e.g., O 2 or N 2 ) or heteronuclear (e.g., CO or NO). Similarly, a triatomic molecule may be homonuclear (e.g., O 3 ) or heteronuclear (e.g., HCN).
The modern concept of the covalent bond has resulted in the ability to predict the geometry and hence the properties of matter such as reactivity, toxicity, and solubility. A fundamental challenge in chemistry is to determine the arrangement of atoms in a molecule in order to elucidate its bonding, geometry, and properties.
Historical Development
Since Roman times matter had been viewed by some as discrete particles somehow linked together. Early in the eighteenth century the behavior of gases was viewed as a function of kinetic theory . Kinetic theory is a group of assumptions to explain the behavior of gases. Among these assumptions are that gases are individual molecules moving in straight lines, that they do not react chemically and occupy essentially no volume compared to the volume between molecules. In 1805 English chemist and physicist John Dalton (1766–1844) proposed that atoms form compounds by joining together in simple, whole numbers. In 1811 Italian chemist Amedeo Avogadro (1776–1856) solidified the distinction between molecules and atoms by proposing that, at constant temperature and pressure, equal volumes of all
gases contain equal numbers of molecules. While Avogadro's theory was published, it was ignored by the scientific community until 1858, when it was revived by Italian chemist Stanislao Cannizzaro (1826–1910), thereby reconciling many inconsistencies chemists were observing. During this same time, valency (the combining capacity of an atom) was defined as the number of hydrogens an atom can combine with.
Initially the structure of molecules was studied using chemical methods, thereby identifying composition, chemical reactions, and the existence of isomers . It was understood that bonds had direction, rigidity, and a certain degree of independence from molecule to molecule. The discovery of the electron in 1897 by English physicist Joseph John Thomson (1856–1940) immediately linked electrons with covalent bonding. Though attacked vigorously for his views, Dutch physical chemist Jacobus Hendricus van't Hoff (1852–1911) discarded the flat-molecule model in favor of geometric relations within each molecule. His brilliant postulate of the tetrahedral arrangement of carbon (proposed simultaneously, but independently, by French chemist Joseph-Achille Le Bel [1847–1930]) was a major breakthrough for chemistry. Later in the nineteenth century the advent of physical methods of investigation led to a great deal of additional information regarding atomic configuration.
Danish physicist Niels Bohr (1885–1962) proposed a quantum theory of the hydrogen atom by suggesting that the electron moves about its nucleus in discrete quanta (the energies of electrons are restricted to having only certain values, quanta, much as stairs do as opposed to a ramp), establishing a balance between the electron's centrifugal force and its attraction for the nucleus. It was not until 1927 that covalent bonding was properly understood, thanks to the contributions of American physical chemist Gilbert N. Lewis (1875–1946), American physicist Edward Uhler Condon (1902–1974), German physicist Walter Heitler (1904–1981), and German physicist Fritz London (1900–1954).
In his 1916 paper The Atom and the Molecule , Lewis proposed that a chemical (covalent) bond between two atoms involves the sharing of electrons between the nuclei. Thus a single bond (for hydrogen, H-H) results when an electron from each atom forms an electron pair that is shared between the two nuclei (H:H); a double bond involves two electrons from each atom (e.g., the carbon-carbon bond in (H:) 2 C::C(:H) 2 ); and a triple bond involves three electrons from each atom (e.g., the carbon-carbon bond in H:C:::C:H). Such representations are referred to as Lewis dot structures. Lewis further postulated that an electron octet (and in a few cases an electron pair) forms a complete shell of electrons with spatial rigidity and chemical inertness—hence a stable arrangement.
American chemist Irving Langmuir (1881–1957) proposed that many chemical facts could be coordinated by applying these new ideas. Others followed by suggesting that a bond is a balance between nucleus-nucleus and electron-electron repulsions and electron-nuclei attractions. American chemist Linus Pauling (1901–1994) assembled these ideas in his seminal book, The Nature of the Chemical Bond.
Valence Shell Electron Pair Repulsion Theory
Molecular geometries are determined by the number and locations of valence electrons around the atoms. Both bonded and lone pair electrons repel each other, staying as far apart as possible, thereby causing the molecule to occupy specific shapes (much as balloons assume fixed arrangements when tied together). These geometries are important in determining chemical properties. One method for determining the structure of covalent molecules is the valence shell electron pair repulsion (VSEPR) method, proposed in 1957 by Canadian chemist Ronald Gillespie and Australian chemist Ronald Nyholm in a classic paper titled "Inorganic Stereochemistry." The theory states that the geometry around a given atom is a function primarily of minimizing the electron pair repulsions. The key postulates of the VSEPR theory are:
- All electrons are negatively charged.
- Bonds are electron groups.
- Lone pair and bonded electrons (and therefore bonds) repel each other.
Geometries of most covalent molecules may be determined by following these steps:
- Determine the central atom. This may be the atom present singly (e.g., B in BF 3 ), the larger atom (e.g., P in POCl 3 ), the atom written in the center (e.g., C in HCN), or the atom with the largest number of bonds (e.g., C in Cl 2 CO).
- Determine the number of bonds needed for each atom to be bonded to the central atom and write the corresponding Lewis dot structure. Thus, for Cl 2 CO, each chlorine needs a single bond and oxygen needs two bonds; the Lewis dot structure would be (Cl:) 2 C::O. Note that a single bond needs a pair of electrons (one group), a double bond needs two pairs (also one group), and a triple bond needs three pairs (still just one group, since it points in one direction only).
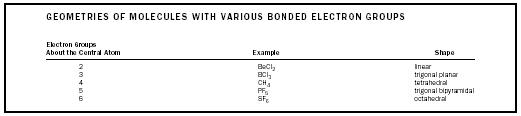
| GEOMETRIES OF MOLECULES WITH VARIOUS BONDED ELECTRON GROUPS | ||
| Electron Groups | ||
| About the Central Atom | Example | Shape |
| 2 | BeCl 2 | linear |
| 3 | BCl 3 | trigonal planar |
| 4 | CH 4 | tetrahedral |
| 5 | PF 5 | trigonal bipyramidal |
| 6 | SF 6 | octahedral |
- Count the total number of bonded and lone pair electron groups about the central atom. For Cl 2 CO it would be three (all bonded) groups. In the case of :NH 3 it would be one lone pair group and three bonded groups for a total of four groups.
- Establish the best electronic (counting all electron groups) and molecular (counting only bonded groups) geometries. Table 1 summarizes this information for bonded groups.
The trigonal bipyramidal shape merits a special note. Contrary to the other shapes, it possesses two types of bonds: the two axial bonds located at 180° from each other, and the three equatorial bonds located perpendicularly to the axis and at 120° from each other.
Each of the examples given in Table 1 has only bonded electrons around its central atom. The existence of lone pair electrons has an effect on the geometry, as seen in Table 2.
For example, water (H 2 O) has two bonded and two lone pair valence electrons about the central atom, oxygen. Its electronic geometry, determined by four total groups, is tetrahedral, and its molecular geometry (meaning the H-O-H shape) is bent. Similarly, the :NH 3 molecule has three
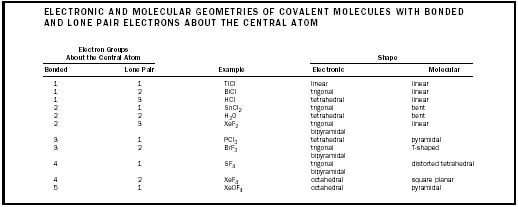
| ELECTRONIC AND MOLECULAR GEOMETRIES OF COVALENT MOLECULES WITH BONDED AND LONE PAIR ELECTRONS ABOUT THE CENTRAL ATOM | ||||
| Electron Groups | ||||
| About the Central Atom | Shape | |||
| Bonded | Lone Pair | Example | Electronic | Molecular |
| 1 | 1 | TlCl | linear | linear |
| 1 | 2 | BiCl | trigonal | linear |
| 1 | 3 | HCl | tetrahedral | linear |
| 2 | 1 | SnCl 2 | trigonal | bent |
| 2 | 2 | H 2 O | tetrahedral | bent |
| 2 | 3 | XeF 2 | trigonal bipyramidal | linear |
| 3 | 1 | PCl 3 | tetrahedral | pyramidal |
| 3 | 2 | BrF 3 | trigonal bipyramidal | T-shaped |
| 4 | 1 | SF 4 | trigonal bipyramidal | distorted tetrahedral |
| 4 | 2 | XeF 4 | octahedral | square planar |
| 5 | 1 | XeOF 4 | octahedral | pyramidal |
bonded and one lone pair electron groups about nitrogen, giving an electronic geometry that is nearly tetrahedral, and a molecular geometry that is pyramidal . Because two bonded pairs repulse less than a bonded pair and a lone pair, which in turn repulse less than two lone pairs, the H-O-H bond angle in water is not 109.5° as expected for a tetrahedron, but 104.5°, with the H-O bonds having been pushed by the lone pairs toward each other. For the trigonal bipyramidal shape, lone pairs always occupy equatorial planar positions. Thus, the molecular geometry of BrF 3 is T-shaped, rather than trigonal planar.
Properties
Both physical and chemical properties are affected by the geometry of a molecule. For instance, the polarity of a molecule is determined by the electronegativity differences of its atoms (electronegativity is the ability of an atom in a molecule to draw electrons toward itself), and the relative geometries of the atoms within the molecule. The molecule BCl 3 , for example, displays a flat triangle (120°) with each Cl atom pulling electrons symmetrically, making the molecule nonpolar . In the pyramidal molecule PCl 3 , however, all chlorines are pulling electrons more or less to one side, making the molecule polar. Since polarity goes hand in hand with solubility, CF 4 is a nonpolar tetrahedral molecule not soluble in water, whereas SF 4 , a distorted tetrahedron, is instantly hydrolyzed by water.
Chemical properties are also very dependent on geometries. For example, in the square planar molecule Pt(NH 3 ) 2 Cl 2 , the chloro (and hence the ammonia) ligands may be placed adjacent to each other (cis isomer), or they may be opposite each other (trans isomer). In addition to having different physical properties, their chemical reactivities are also quite remarkable. The cis isomer is an effective treatment of testicular, ovarian, and certain other cancers, whereas the trans isomer is ineffective. Similarly, the linear, nonpolar CO 2 molecule is inert, whereas the polar CO molecule is a poison.
Other Theories
The VSEPR theory allows chemists to successfully predict the approximate shapes of molecules; it does not, however, say why bonds exist. The quantum mechanical valence bond theory, with its overlap of atomic orbitals , overcomes this difficulty. The resulting hybrid orbitals predict the geometries of molecules. A quantum mechanical graph of radial electron density (the fraction of electron distribution found in each successive thin spherical shell from the nucleus out) versus the distance from the nucleus shows maxima at certain distances from the nucleus—distances at which there are higher probabilities of finding electrons. These maxima correspond to Lewis's idea of shells of electrons.
This theory, however, treats electrons as localized, does not account for unpaired electrons, and does not give information on bond energies. The molecular orbital theory attempts to solve these shortcomings by considering nuclei arranged as in a molecule and determining the resulting molecular orbitals when electrons are fed in one by one.
The electronic and molecular geometries of covalent molecules, and hence their resulting polarities, can thus be predicted fairly accurately. Armed with these tools, one can predict whether or not a molecule should be soluble, reactive, or even toxic.
SEE ALSO Bonding ; Avogadro, Amedeo ; Bohr, Niels ; Cannizzaro, Stanislao ; Dalton, John ; Le Bel, Joseph-Achille ; Lewis, Gilbert N. ; Lewis Structures ; Pauling, Linus ; Thomson, Joseph John ; VAN'T Hoff, Jacobus .
Erwin Boschmann
Bibliography
Atkins, Peter W. (1996). Molecules. New York: W. H. Freeman and Company.
Gillespie, R. J., and Nyholm, R. S. (1957). "Inorganic Stereochemistry." Quarterly Reviews (London) 11:339–380.
Lewis, Gilbert N. (1916). "The Atom and the Molecule." Journal of the American Chemical Society 38:762–786.
Pauling, Linus (1960). The Nature of the Chemical Bond. Ithaca, NY: Cornell University Press.
Pfennig, Brian W., and Frock, Richard L. (1999). "The Use of Molecular Modeling and VSEPR Theory in the Undergraduate Curriculum to Predict the Three-Dimensional Structure of Molecules." Journal of Chemical Education 76(7):1018–1022.
Comment about this article, ask questions, or add new information about this topic: