Ascorbic Acid
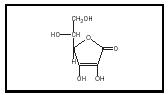
Ascorbic acid or vitamin C is an antiscorbutic agent. Scurvy is a disease that potentially ranks as the second most important nutritional deficiency, after protein-calorie malnutrition. Scurvy, once common in among sailors, causes bleeding and inflamed gums, loose teeth, poor wound healing, pain in the joints, muscle wasting, etc. The structure of vitamin C is simple (see Figure 1), resembling a monosaccharide, and most animals are able to synthesize ascorbic acid. Only primates, guinea pigs, and some fruit bats have lost the ability to synthesize it.
Vitamins are organic molecules that mainly function as catalysts for reactions in the body. A catalyst is a substance that allows a chemical reaction to occur using less energy and less time than it would take under normal conditions.
Vitamin C is water-soluble and very important to all humans because it is vital to the production of collagen. Inside the cell, it helps form a precursor molecule called "procollagen" that is later packaged and modified into collagen outside the cell. Collagen is a gluelike substance that binds cells together to form tissues. It is the most abundant of the fibers contained in connective tissues. Connective tissue gives the human body form and supports its organs.
Vitamin C is also important as it helps protect the fat-soluble vitamins A and E, as well as fatty acids from oxidation . It is therefore a reducing agent and scavenger of radicals (sink of radicals). Radicals, molecules with unpaired electrons, are very harmful to the body as a result of their high reactivity, which may induce mutations and possibly cancer. Vitamin C, being an excellent source of electrons, can therefore donate electrons to free radicals such as hydroxyl and superoxide and quench their reactivity.
A debate exists over the anticancer properties of vitamin C. However, current evidence suggests that the major benefit of ascorbic acid with regard to cancer may be in reducing the risk of developing cancer, rather than in therapy. Vitamin C can work inside the cells to protect DNA (deoxyribonucleic acid), the hereditary material in cells, from the damage caused by free radicals. Also, it can reduce the development of nitrosamines (amines linked to the NO group) from nitrates, chemicals that are commonly used in processed foods. Once formed, nitrosamine can become carcinogenic (cancer-causing).
Sources of vitamin C are numerous: citrus fruits such as oranges, limes, and grapefruits and vegetables including tomatoes, green peppers, potatoes, and many others. The recommended dietary allowance (RDA) of vitamin C is 60 milligrams (0.0021 ounces) per day. An average American ingests about 72 milligrams (0.0025 ounces) a day. Some studies suggest higher daily doses especially for the elderly, women, and the infirm. For example, the late Linus Pauling, best known for his theory on chemical bonding and a two-time Nobel Prize winner, consumed several grams of vitamin C per day for the last forty years of his life and lived to age ninety-three.
SEE ALSO Catalysis and Catalysts ; Pauling, Linus .
Joseph Bariyanga
Bibliography
Meisenberg, Gerhard, and Simmons, William H. (1998). Principles of Medical Biochemistry. St Louis, MO: Mosby.