Artificial Sweeteners

There are presently four artificial, or synthetic, sweeteners that have been approved by the U.S. Food and Drug Administration (FDA): saccharin, aspartame, acesulfame-K, and sucralose. People use artificial sweeteners because they suffer from diseases such as diabetes mellitus, because they are concerned about dental caries and periodontal disease, or because they wish to lose or to avoid gaining weight. Artificial sweeteners in very small quantities give foods sweetness, and most are not metabolized, meaning that the artificial sweeteners themselves furnish zero dietary calories.
Sweetener Molecules and Sweetness
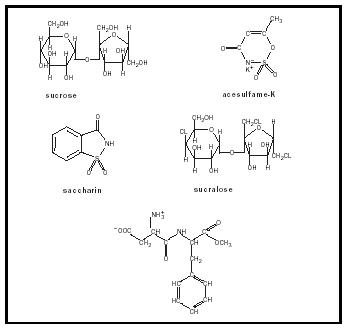
Sucrose and most artificial sweeteners are chemically quite dissimilar. Sucrose (C 12 H 22 O 11 ), the most common "natural" sweetener, is a disaccharide composed of the monosaccharides glucose and fructose. Saccharin has the formula C 7 H 5 O 3 NS. Aspartame (C 13 H 18 O 5 N 2 ), L-aspartyl-L-phenylalanine methyl ester , is the methyl ester of a dipeptide. Acesulfame-K has the formula C 5 H 6 O 3 NS. Sucralose (C 11 H 19 O 8 Cl 3 ) is prepared from sucrose via the substitution of three chloride groups for three hydroxyl groups. The molecular structures of sucrose, saccharin, aspartame, acesulfame-K, and sucralose are shown in Figure 1.
A sweetener must be soluble in water and the molecule must bind readily to a specific kind of receptor molecule at the surface of the tongue. The receptor is coupled to a G-protein, which dissociates when the sweetener binds to the receptor, activating a nearby enzyme, and triggering a sequence of events resulting in signals that are carried to and interpreted by the brain. The sweetness "signal" depends on this interaction between receptor and sweetener. The importance of molecular shape to sweetness is illustrated by the case of aspartame, as its stereo isomer , L-aspartyl-D-phenylalanine methyl ester, has a bitter, not a sweet, taste.
Discovery, Sweetness, and Metabolic Products
Saccharin was the first artificial sweetener, discovered in 1879 by Constantin Fahlberg at Johns Hopkins University. The Monsanto Chemical Works was incorporated in 1901 to produce saccharin in the United States. Saccharin is easy to make, stable when heated, and is approximately 300 times sweeter than sucrose when equal quantities are compared. One common saccharin product is Sweet and Low.
Saccharin does not accumulate in body tissues. Controversy over the use of saccharin has existed for over a century. In the 1960s and early 1970s saccharin and/or its impurities were shown to cause bladder cancer in rats.
In 1977 a Canadian study concluded that saccharin was the causative agent. Saccharin was banned in Canada. At about the same time the FDA proposed to limit the use of saccharin, but public outcry was so great that the U.S. Congress placed a moratorium on bans of saccharin until further studies were completed. The original moratorium was in effect for two years but has been continually extended to the present day.
Aspartame was discovered in 1965 by James Schlatter at G.D. Searle & Company. Aspartame is relatively easy to make and is approximately 200 times sweeter than sucrose. It is most commonly sold as Nutra Sweet and Equal. It is less stable than saccharin and breaks down above 29.44°C (85°F). In the body, aspartame is broken down into/absorbed as products that include aspartate, phenylalanine, and methanol. Phenylalanine is toxic to individuals who are homozygous (having identical genes in homologous chromosomes) for phenylketonuria, a genetic disease wherein individuals cannot catabolize phenylalanine. Phenylketonuria causes mental retardation. Products containing aspartame must therefore be labeled for phenylalanine. The FDA considers aspartame to be one of the most thoroughly studied and tested food additives and has judged it to be safe. Controversy still lingers with respect to the effects of aspartame's breakdown products—phenylalanine and aspartate, as well as methanol and its breakdown products formaldehyde and formate.
Acesulfame-K was discovered in 1967 by scientists working at Hoechst AG. It is also called Sunett. It is approximately 200 times sweeter than sugar. It has a long shelf life and does not break down in foods that are cooked or

baked. Over ninety studies have been completed that have concluded that acesulfame-K is safe.
Sucralose was discovered in 1976 by researchers at Tate & Lyle PLC. It is also called Splenda. Sucralose is approximately 600 times sweeter than sugar and is stable at high temperatures. It was approved by the FDA in 1998–1999, and it is supported by a safety database of more than 110 studies. Concerns persist, including concerns over possible side effects associated with breakdown products (which include chlorine and 1,6-dichlorofructose), shrunken thymus glands (and their impacts on the immune system), and unanticipated effects that may not have manifested during the short time that sucralose has been used.
SEE ALSO Toxicity .
Vivienne A. Whitworth
Bibliography
Henkel, John (1999). "Sugar Substitutes: Americans Opt for Sweetness and Lite." FDA Consumer Magazine 33(6):12–16.
Kretchmer, Norman, and Hollenbeck, Claire B., eds. (1991). Sugars and Sweeteners. Boca Raton, FL: CRC Press.
Smith, David V., and Margolskee, Robert F. (2001). "Making Sense of Taste." Scientific American 284(3):32–39.
Internet Resources
Hodgin, Greg. "The History, Synthesis, Metabolism, and Uses of Artificial Sweeteners." Available from http://www.ecit.emory.edu/ecit .
Kitts, David D. "Sweetness Chemistry." Available from http://www.agsci.ubc.ca/courses .
Lok, Corie. "Sweet Tooth Gene Found." Available from http://www.nature.com/nsu/ .
Tate & Lyle PLC. "Sucralose—Technical Information." Available from http://www.officialsucralosesite.com .