ZIRCONIUM

Overview
Compounds of zirconium have been known for centuries. Yet, the element itself was not recognized until 1789. In that year, German chemist Martin Heinrich Klaproth (1743-1817) discovered the element in a stone brought to him from the island of Ceylon (now Sri Lanka).
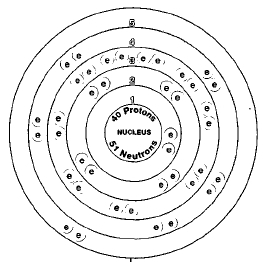
Zirconium is one of the transition metals. The transition metals are the elements found in Rows 4 through 7 and between Groups 2 and 13 in the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. Zirconium is located below titanium, which it resembles, in the periodic table. Below zirconium is hafnium, a chemical twin of zirconium.
An important use of zirconium is in nuclear power plants. Its most important compound is zircon, which has a number of industrial applications. Zircon can also be obtained in gemstone quality. A gemstone is a mineral that can be cut and polished and used in jewelry or art.
SYMBOL
Zr
ATOMIC NUMBER
40
ATOMIC MASS
91.22
FAMILY
Group 4 (IVB)
Transition metal
PRONUNCIATION
zir-KO-nee-um
Discovery and naming
Naturally occurring compounds of zirconium have been used by humans since before the birth of Christ. For example, St. John talks about the jacinth (or hyacinth) stone. He says it was one of the jewels found in the walls surrounding Jerusalem. The jacinth stone was the same mineral referred to by the Persians as zargun, meaning "gold-like" in Persian.
Early chemists did not study the jacinth stone very carefully. They thought it was another form of alumina (aluminum oxide). Alumina was a well-known mineral at the time. In fact, it was not until Klaproth undertook the study of the jacinth stone that he realized it contained a new element. Klaproth at first referred to the stone as Jargon of Ceylon. When he knew that he had found a new element, he suggested the name zirconium for it.
The material discovered by Klaproth was not a pure element. Instead, it
was a compound of zirconium and
oxygen,
zirconium oxide (ZrO
2
). The pure metal was not produced until 1824 when Swedish chemist Jons
Jakob Berzelius (1779-1848) made fairly pure zirconium. He made the metal
by heating a mixture of
potassium
and potassium zirconium fluoride (ZrK
2
F
6
):
Physical properties
Zirconium is a hard, grayish-white, shiny metal. Its surface often has a flaky-like appearance. It also occurs in the form of a black or bluish-black powder. It has a melting point of 1,857°C (3,375°F) and a boiling point of 3,577°C (6,471°F. Its density is 6.5 grams per cubic centimeter.
Zirconium has one physical property of special importance: It is transparent to neutrons. Neutrons are tiny particles with no charge in the nucleus (center) of almost all atoms. Industrially, they are used to make nuclear fission reactions occur. Nuclear fission is the process in which large atoms break apart. Large amounts of energy and smaller atoms are produced during fission. Fission reactions are used to provide the power behind nuclear weapons (such as the atomic bomb). They are also used to produce energy in a nuclear power plant.
One of the difficult problems in building a nuclear power plant is selecting the right materials. Many metals capture neutrons that pass through them. The neutrons become part of the metal atoms and are no longer available to make fission reactions
Zirconium is one of the best of these metals. If zirconium is used to make the parts in a nuclear power plant, it will not remove neutrons from the fission reaction going on inside the plant.
A special alloy of zirconium has been developed just for this purpose. It is called Zircaloy. The manufacture of Zircaloy accounts for 90 percent of the zirconium metal used in the world today.
Chemical properties
Zirconium is a fairly inactive element. When exposed to air, it reacts with oxygen to form a thin film of zirconium oxide (ZrO 2 ). This film protects the metal from further corrosion (rusting). Zirconium does not react with most cold acids or with water. It does react with some acids that are very hot, however.
Occurrence in nature
Zirconium is a fairly common element in the Earth's crust. Its abundance is estimated to be 150 to 230 parts per million.
That places it just below carbon and sulfur among elements occurring in the Earth's crust.
The two most common ores of zirconium are zircon, or zirconium silicate (ZrSiO 4 ); and baddeleyite, or zirconia or zirconium oxide (ZrO 2 ). The amount of zirconium produced in the United States is not reported. That information is regarded as a trade secret. The largest suppliers of zirconium minerals in the world are Australia and South Africa. These two countries produce about 85 percent of the world's zirconium.
Isotopes
There are five naturally occurring isotopes of zirconium: zirconium-90, zirconium-91, zirconium-92, zirconium-94, and zirconium-96. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
About a dozen radioactive isotopes of zirconium are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
No radioactive isotope of zirconium has any important practical application.
Extraction
Zirconium ores are first converted to zirconium tetrachloride (ZrCl
4
). This compound is then mixed with magnesium metal at high temperature:
Naturally occurring zircon is in demand as a gemstone. It is polished, cut, and used for jewelry and art.
Uses
Many zirconium alloys are available. They are used to make flash bulbs, rayon spinnerets (the nozzles from which liquid rayon is released), lamp filaments, precision tools, and surgical instruments. These uses make up only a small amount of the
Compounds
About 95 percent of all zirconium produced is converted into a compound before being used. The two most common compounds made are zircon (zirconium silicate) and zirconia (zirconium oxide).
Naturally occurring zircon is in demand as a gemstone. It is polished, cut, and used for jewelry and art. Natural zircon often includes uranium, thorium, and other radioactive elements. The presence of these elements often gives a zircon a special brilliance and fire-like quality, resembling fine diamonds.
Zircon has other properties that make it desirable in industrial applications. For example, it is an excellent refractory material. A refractory is a material that does not conduct heat well. It is able to withstand very high temperatures without cracking or breaking down.
Zircon is used to make the foundry molds used to make metal pieces of all shapes. Molten metal is poured into the mold. When it cools, it is removed from the mold. The use of zircon in a refractory mold produces a smooth surface on the metal.
Zircon is also used to make bricks in high-temperature furnaces and ovens. These furnaces and ovens are used to work with molten metals. Zircon bricks are ideal for such ovens because they reflect heat and are not destroyed by high temperatures.
Both zircon and zirconia are used as abrasives. An abrasive is a powdery material used to grind or polish other materials. Another important use of zircon and zirconia is in making objects opaque. Opaque means that light is not able to pass through. Suppose a person wants to make a glaze for pottery that looks completely white. The glaze must reflect all light that strikes it and not let any light through. Adding zircon or zirconia to the glaze will achieve this result.
Zirconium can cause skin irritation. Deodorant products containing zirconium have been found to produce skin rashes.
Health effects
Zirconium is regarded as relatively safe. Some studies have shown that it can cause skin irritation, however. Deodorant products containing zirconium have been found to produce skin rashes.