BROMINE

Overview
Bromine is a member of the halogen family. Halogens are the elements that make up Group 17 (VIIA) of the periodic table. The periodic table is a chart that shows how elements are related to one another. The halogens are also known as the salt formers. Fluorine, chlorine, bromine, iodine, and astatine form salts when chemically combined with a metal.
Bromine was discovered, at almost the same time in 1826, by two men, German chemist Carl Lowig (1803-90) and French chemist Antoine-Jerome Balard (1802-76). While Balard announced his discovery first, Lowig had simply not completed his studies of the element when Balard made his announcement.
SYMBOL
Br
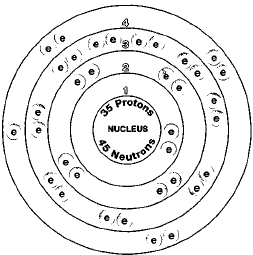
ATOMIC NUMBER
35
ATOMIC MASS
79.904
FAMILY
Group 17 (VIIA)
Halogen
PRONUNCIATION
BRO-meen
Nearly 90 percent of all bromine produced comes from the United States, Israel, or the United Kingdom. In 1996, about 450,000,000 kilograms (one billion pounds) of the element were produced worldwide. The largest single use of the element is in the manufacture of flame retardants. Flame retardants are chemicals added to materials to prevent burning or to keep them from burning out of control. Other major uses are in the manufacture of drilling fluids, pesticides, chemicals for the purification of water, photographic chemicals, and as an additive to rubber.
Discovery and naming
Compounds of bromine had been known for hundreds of years before the element was discovered. One of the most famous of these compounds was Tyrian purple, also called royal purple. (Tyrian comes from the word Tyre, an ancient Phoenician city.) Only very rich people or royalty could afford to buy fabric dyed with Tyrian purple. It was obtained from a mollusk (shell fish) found on the shores of the Mediterranean Sea (a large body of water bordered by Europe, Asia, and Africa).
In 1825, Löwig enrolled at the University of Heidelberg in Germany to study chemistry. He continued an experiment he had begun at home in which he added chlorine to spring water. The addition of ether to that mixture produced a beautiful red color. Löwig suspected he had discovered a new kind substance. A professor encouraged him by suggesting he study the substance in more detail.
As these studies progressed, Balard published a report in a chemical journal that announced the discovery of the new element bromine. The element had all the properties of Löwig's new substance. The two chemists had made the discovery at nearly the same time! Balard, however, is credited as the discoverer of bromine, because scientists acknowledge the first person to publish his or her findings.
In Greek, the word bromos means "stench" (strong, offensive odor). Bromine lives up to the description. The odor is intense and highly irritating to the eyes and lungs.
Chemists found that bromine belonged in the halogen family. They knew that it had properties similar to other halogens and placed it below fluorine and chlorine in the periodic table.
Physical properties
Only two liquid elements exist—bromine and mercury. At room temperature, bromine is a deep reddish-brown liquid. It evaporates easily, giving off strong fumes that irritate the throat and lungs. Bromine boils at 58.8°C (137.8°F), and its density is 3.1023 grams per cubic centimeter. Bromine freezes at -7.3°C (18.9°F).
Bromine dissolves well in organic liquids—such as ether, alcohol, and carbon tetrachloride—but only slightly in water. Organic compounds contain the element carbon.
Chemical properties
Bromine is a very reactive element. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. It reacts with many metals, sometimes very vigorously. For instance, with potassium, it reacts explosively. Bromine even combines with relatively unreactive metals, such as platinum and palladium.
Occurrence in nature
Bromine is too reactive to exist as a free element in nature. Instead, it occurs in compounds, the most common of which are sodium bromide (NaBr) and potassium bromide (KBr). These compounds are found in seawater and underground salt beds. These salt beds were formed in regions where oceans once covered the land. When the oceans evaporated (dried up), salts were left behind—primarily sodium chloride (NaCl), potassium chloride (KCl), and sodium and potassium bromide. Later, movements of the Earth's crust buried the salt deposits. Now they are buried miles underground. The salts are brought to the surface in much the same way that coal is mined.
Bromine is a moderately abundant element. Its abundance in the Earth's crust is estimated to be about 1.6 to 2.4 parts per million. It is far more abundant in seawater where it is estimated at about 65 parts per million.
In some regions, the abundance of bromine is even higher. For example, the Dead Sea (which borders Israel and Jordan), has a high level of dissolved salts. The abundance of bromine there is estimated to be 4,000 parts per million. The salinity, or salt content, is so high that nothing lives in the water. This is why it is called the Dead Sea.
Isotopes
Two naturally existing isotopes of bromine exist, bromine-79 and bromine-81. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
At least 16 radioactive isotopes of bromine are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
No isotope of bromine has any important commercial use.
The salinity, or salt content, is so high that nothing lives in the water. This is why it is called the Dead Sea.
Extraction
The method used by Lowig and Balard to collect bromine continues to be
used today. Chlorine is added to seawater containing sodium bromide or
potassium bromide. Chlorine is more active than bromine and replaces
bromine in the reaction:
Uses
The most important use of bromine today is in making flame retardant materials. Many materials used in making clothing, carpets, curtains, and drapes are flammable, and if a flame touches them, they burn very quickly. Chemists have learned how to make materials more resistant to fires by soaking them in a bromine compound. The compound coats the fibers of the material. The bromine compound can also be chemically incorporated into the material.
The bromine compounds used in flame retardants are often complicated. One such compound is called tris(dibromopropyl)phosphate ((Br 2 C 3 H 5 O) 3 PO). However, this compound has been found to be a carcinogen (cancer-causing substance). Its use, therefore, has been severely restricted.
About 20 percent of all bromine is used in drilling wells. Calcium bromide (CaBr 2 ), sodium bromide (NaBr), or zinc bromide (ZnBr 2 ) are added to the well to increase the efficiency of the drilling process.
Bromine is also important in the manufacture of pesticides, chemicals used to kill pests. Methyl bromide (CH 3 Br) has been used for years to treat crop lands. Methyl bromide is sprayed on the surface or injected directly into the ground.
Some methyl bromide always evaporates into the air where it damages the ozone layer. Ozone (O 3 ) gas filters out a portion of the ultraviolet (UV) radiation from the sun. UV radiation causes skin cancer, sunburn, and damage to plants and fragile organisms.
Worldwide production of methyl bromide will end in 2001 because of its effect on the ozone layer. The United States plans to stop production of the compound even earlier. Farmers believe nothing works as well as methyl bromide in eliminating certain pests. They are concerned that crop production will suffer if methyl bromide is banned.
The most important use of bromine today is in making flame retardant materials.
Ethylene dibromide (C 2 H 4 Br 2 ) is a bromide compound added to leaded gasoline. The lead in "leaded gasoline" is tetraethyl lead (Pb(C 2 H 5 )) 4 ). It helps fuels burn more cleanly and keeps car engines from "knocking." "Knocking" is a repetitive metallic banging sound that occurs when there are ignition problems

But leaded gasoline gives off free lead as it burns. Free lead is a very toxic element that causes damage to the nervous system. Ethylene dibromide is added to react with free lead and convert it to a safe compound.
Ethylene dibromide does not completely solve the problem. Some free lead still escapes into the atmosphere. Leaded gasoline has been banned in the United States for many years but is still used in other countries.
The most popular element for purifying public water supplies and swimming pools used to be chlorine. Bromine compounds have become more popular for their superior bacteria killing power.
Health effects
Bromine is toxic if inhaled or swallowed. It can damage the respiratory system and the digestive system, and can even cause death. It can also cause damage if spilled on the skin.
Comment about this article, ask questions, or add new information about this topic: