ARSENIC
Overview
Arsenic compounds have been known since at least the days of Ancient Greece and Rome (thousands of years ago). They were used by physicians and poisoners. The compound most often used for both purposes was arsenic sulfide (As 2 3 ).
Arsenic was first recognized as an element by alchemists. Alchemy was a kind of pre-science that existed from about 500 B.C. to about the end of the 16th century. People who studied alchemy—alchemists—wanted to find a way of changing lead, iron, and other metals into gold. They were also looking for a way to have eternal life. Alchemy contained too much magic and mysticism to be a real science, but alchemists developed a number of techniques and produced many new materials that were later found to be useful in modern chemistry.
SYMBOL
As
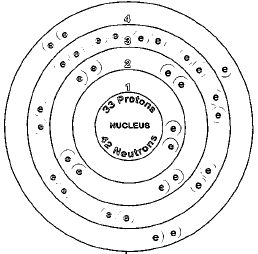
ATOMIC NUMBER
33
ATOMIC MASS
74.9216
FAMILY
Group 15 (VA)
Nitrogen
PRONUNCIATION
AR-se-nick
A small amount of arsenic is used in alloys. An alloy is made by melting and then mixing two or more metals. The mixture has properties different from those of individual metals. The most important use of arsenic in the United States is in wood preservatives.
Discovery and naming
Arsenic can be produced from its ores very easily, so many early craftspeople may have seen the element without realizing what it was. Since arsenic is somewhat similar to mercury, early scholars probably confused the two elements with each other.
Credit for the actual discovery of arsenic often goes to alchemist Albert the Great (Albertus Magnus, 1193-1280). He heated a common compound of arsenic, orpiment (As 2 S 3 ), with soap. Nearly pure arsenic was formed in the process.
By the mid-seventeenth century, arsenic was well known as an element. Textbooks often listed methods by which the element could be made from its compounds.
Physical properties
Arsenic occurs in two allotropic forms. Allotropes are forms of an element with different physical and chemical properties. The more common form of arsenic is a shiny, gray, brittle, metallic-looking solid. The less common form is a yellow crystalline solid. It is produced when vapors of arsenic are cooled suddenly.
When heated, arsenic does not melt, as most solids do. Instead, it changes directly into a vapor (gas). This process is known as sublimation. However, under high pressure, arsenic can be forced to melt at about 814°C (1,500°F). Arsenic has a density of 5.72 grams per cubic centimeter.
Chemical properties
Arsenic is a metalloid. A metalloid is an element that has properties of both metals and non-metals. Metalloids occur in the periodic table on either side of the staircase line that starts between boron and aluminum.
When heated in air, arsenic combines with oxygen to form arsenic oxide (As 2 O 3 ). A blue flame is produced, and arsenic oxide can be identified by its distinctive garlic-like odor.
Arsenic combines with oxygen more slowly at room temperatures. The thin coating of arsenic oxide that forms on the element prevents it from reacting further. Arsenic does not dissolve in water or most cold acids. It does react with some hot acids to form arsenous acid (H 3 AsO 3 ) or arsenic acid (H 3 AsO 4 ).
Occurrence in nature
Arsenic rarely occurs as a pure element. It is usually found as a compound. The most common ores of arsenic are arsenopyrite (FeAsS), orpiment (As 2 S 3 ), and realgar (As 4 S 4 ). These compounds are obtained as a by-product of the mining and purification of silver metal.
The abundance of arsenic in the Earth's crust is thought to be about 5 parts per million. That places it among the bottom third of the elements in abundance in the Earth's crust.
The world's largest producers of arsenic are China, Chile, Mexico, Belgium, Namibia, and the Philippines. The United States does not produce any arsenic.
Isotopes
One naturally occurring isotope of arsenic exists, arsenic-75. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
About 14 radioactive isotopes of arsenic are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
None of the isotopes of arsenic have any important commercial use.
Extraction
The process of recovering arsenic from its ores is a common one used with
metals. The ore is first roasted (heated in air) to chemically convert
arsenic sulfide to arsenic oxide. The arsenic oxide is then heated with
charcoal (pure
carbon
). The carbon reacts with the oxygen in arsenic oxide, leaving behind pure
arsenic:

Uses
Arsenic is mostly used in compounds. A much smaller amount of the element itself is used in alloys. For example, certain parts of lead storage batteries used in cars and trucks contain alloys of lead and arsenic. Arsenic has also been used to make lead shot in the past. The amount of arsenic used in these applications is likely to continue to decrease. It is too easy for arsenic to get into the environment from such applications.
Minute amounts of arsenic are used in the electronics industry. It is added to germanium and silicon to make transistors. A compound of arsenic, gallium arsenide (GaAs), is also used to make light-emitting diodes (LEDs). LEDs produce the lighted numbers in hand-held calculators, clocks, watches, and a number of other electronic devices.
Compounds
Arsenic has a fascinating history as a healer and killer. Early physicians, such as Hippocrates (c. 460 B.C -370 B.C. ) and Paracelsus (1493-1541), recommended arsenic for the treatment of some diseases. In more recent times, compounds of arsenic have been used to treat a variety of diseases, including syphilis and various tropical diseases.
Arsenic has a special place in the history of modern medicine. In 1910, German biologist Paul Ehrlich (1854-1915) invented the first drug that would cure syphilis, a sexually transmitted disease. This drug, called salvarsan, is a compound of arsenic. Its chemical name is arsphenamine.
Was a U.S. president poisoned?

O n July 9, 1850, the twelfth president of the United States, Zachary Taylor (1784-1850), died in office. He had served as president for a little more than sixteen months. The cause of death was widely reported as gastroenteritis (an inflammation in the stomach and intestines). He had gotten sick after eating a mixture of cherries and buttermilk. But for years, historians wondered whether Taylor had been murdered—poisoned by arsenic!
On June 17, 1991, Taylor's remains were exhumed (removed from his grave) from a cemetery in Louisville, Kentucky. The late president's descendents agreed with historians that the possibility of poisoning existed. Samples of Taylor's hair and fingernails were taken to Oak Ridge National Laboratory, in Oak Ridge, Tennessee, for analysis.
Scientists used a process that measured the amount of arsenic in the tissue samples. Most human bodies do contain traces of arsenic. So the key issue was whether there would be more arsenic in the tissue samples than would be normal for someone who had been dead for 141 years. If there were, that would mean Taylor was probably poisoned; if not, death by natural causes was more likely.
The Kentucky medical examiner (a public official who studies corpses to find the cause of death) came to a conclusion. He said the amount of arsenic found in Taylor's samples was several hundred times less than what could be expected had the president been poisoned by arsenic. So while some still wonder whether Taylor was poisoned, arsenic was certainly not the chemical element used. And, more than likely, it was the cherries and buttermilk!
Compounds of arsenic have long been used for less happy purposes. Especially during the Middle Ages, they were a popular form of committing murder. At the time, it was difficult to detect the presence of arsenic in the body. A person murdered by receiving arsenic was often thought to have died of pneumonia.
The toxic properties of arsenic compounds made them useful as rat poison. However, they are seldom used for this purpose today. Safer compounds are used that do not present a threat to humans, pets, and the environment.
Today, the most important use of arsenic is in the preservation of wood. It is used in the form of a compound called chromated copper arsenate (CCA). CCA accounts for about 90 percent of all the arsenic used in the United States. It is added to wood used to build houses and other wooden structures. It prevents organisms from growing in the wood and causing it to rot. In 1996, about 19,200,000 metric tons of arsenic were used for this purpose. There is significant concern about the use of arsenic-treated wood in playground equipment and raised garden beds because of toxicity.
Health effects
Arsenic and its compounds are toxic to animals. In low doses, arsenic produces nausea, vomiting, and diarrhea. In larger doses, it causes abnormal heart beat, damage to blood vessels, and a feeling of "pins and needles" in hands and feet. Small corns or warts may begin to develop on the palms of the hands and the soles of the feet. Direct contact with the skin can cause redness and swelling.
The most important use of arsenic is in the preservation of wood.
Long term exposure to arsenic and its compounds can cause cancer. Inhalation can result in lung cancer. If swallowed, cancer is likely to develop in the bladder, kidneys, liver, and lungs. In large doses, arsenic and its compounds can cause death.