Nicotinamide Adenine Dinucleotide
Nicotinamide adenine dinucleotide (NAD) is the coenzyme form of the vitamin niacin. Most biochemical reactions require protein catalysts (enzymes). Some enzymes, lysozyme or trypsin, for example, catalyze reactions by themselves, but many require helper substances such as coenzymes, metal ions, and ribonucleic acid (RNA). Niacin is a component of two coenzymes: NAD, and nicotinamide adenine dinucleotide phosphate (NADP). NAD + (the oxidized form of the NAD coenzyme) is important in catabolism and in the production of metabolic energy. NADP + (the oxidized form of NADP) is important in the biosynthesis of fats and sugars.
Hans von Euler is generally recognized as the first to establish the chemical structure of NAD (Metzler, p. 468). Von Euler and Arthur Harden shared the 1929 Nobel Prize in physiology or medicine for the discovery of coenzymes (including NAD). Later von Euler showed that NAD contains two units of the sugar ribose, two phosphate groups, one adenine unit, and
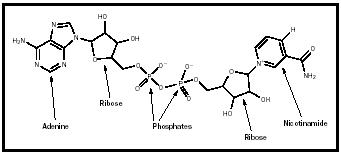
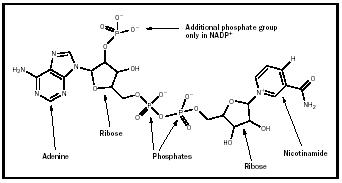
one nicotinamide unit (derived from niacin). (See Figure 1a.) The adenine, ribose, and phosphate compounds are linked exactly as in the nucleotide molecule adenosine diphosphate (ADP). In the case of NAD + , the nicotinamide ring has a positive charge on its nitrogen atom: This is the + indicated in the designation NAD + . This is often confusing, because the molecule as a whole is negatively charged due to the presence of the phosphate groups, as shown in the figure. In 1934 Otto Warburg and William Christian discovered a variant of NAD + in human red blood cell extracts (Metzler, p. 466). This form, called NADP + , contains a third phosphate group attached to one of the ribose rings (see Figure 1b).
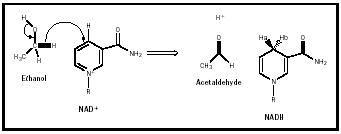
NAD + and NADP + play an essential role in many biochemical reactions, especially redox reactions in which oxidoreductase enzymes transfer hydrogen. (See Table 1 for a partial list of enzymes that require NAD + and NADP + .) The redox reaction shown in Figure 2 is catalyzed by the oxidoreductase enzyme alcohol dehydrogenase. In this reaction, two hydrogen atoms and two electrons (the two electrons of the C-H bond) are removed from the ethanol molecule. One hydrogen atom and both electrons, shown in red, are transferred to NAD + , generating NADH. (A molecule's acquisition of electrons is called reduction, thus NADH is the reduced form of NAD + . Conversely, NAD + is the oxidized form, the form with fewer electrons.) NADP + can be reduced to NADPH, just as NAD + can be reduced to NADH, although different enzymes will be involved. Enzymes that use NAD + rarely use NADP + and vice versa, making it possible to separate the
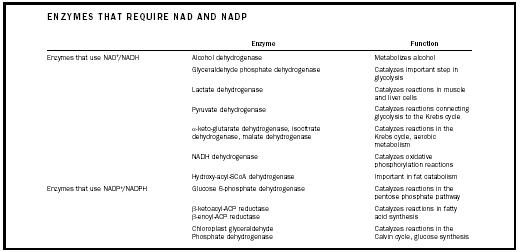
| ENZYMES THAT REQUIRE NAD AND NADP | ||
| Enzyme | Function | |
| Enzymes that use NAD + /NADH | Alcohol dehydrogenase | Metabolizes alcohol |
| Glyceraldehyde phosphate dehydrogenase | Catalyzes important step in glycolysis | |
| Lactate dehydrogenase | Catalyzes reactions in muscle and liver cells | |
| Pyruvate dehydrogenase | Catalyzes reactions connecting glycolysis to the Krebs cycle | |
| α -keto-glutarate dehydrogenase, isocitrate dehydrogenase, malate dehydrogenase | Catalyzes reactions in the Krebs cycle, aerobic metabolism | |
| NADH dehydrogenase | Catalyzes oxidative phosphorylation reactions | |
| Hydroxy-acyl-SCoA dehydrogenase | Important in fat catabolism | |
| Enzymes that use NADP + /NADPH | Glucose 6-phosphate dehydrogenase | Catalyzes reactions in the pentose phosphate pathway |
| β -ketoacyl-ACP reductase β -enoyl-ACP reductase | Catalyzes reactions in fatty acid synthesis | |
| Chloroplast glyceraldehyde Phosphate dehydrogenase | Catalyzes reactions in the Calvin cycle, glucose synthesis | |
biosynthetic and energy-producing functions of NADP + and NAD + . NAD + and NADP + act as electron acceptors in oxidoreductase catalyzed reactions; NADH and NADPH act as electron donors.
The transfer of hydrogen to NAD + is stereospecific , and dehydrogenases are now classified as H A side or H B side enzymes, according to the "side" of the NAD molecule they act on. Alcohol dehydrogenase, which catalyzes the reaction shown in Figure 2, is an H A side enzyme, as it promotes the transfer of hydrogen from ethanol to the "A position" of NADH. This specificity was somewhat unexpected, as the flat nicotinamide group can be approached (by a dehydrogenase enzyme) equally well from either side in solution, when NAD + is bound to the enzyme; however, one side or the other of NAD is more approachable and therefore preferred.
Both NADH and NADPH have a distinctive signal in ultraviolet spectroscopy . This signal is lost when NADH or NADPH is oxidized (to NAD + or NADP + ). This phenomenon has been employed by thousands of scientists to monitor a wide variety of enzyme-catalyzed reactions.
SEE ALSO Coenzyme ; Enzymes ; Nicotinamide .
David Speckhard
Bibliography
Magill, Frank (1991). The Nobel Prize Winners: Physiology or Medicine, Vol. 1. Pasadena, CA: Salem Press.
Metzler, David E. (1977). Biochemistry: The Chemical Reactions of Living Cells. New York: Academic Press.