Iridium

MELTING POINT:
2,454°C
BOILING POINT:
3,590°C
DENSITY:
22.6 g/cm
3
(at 25°C)
MOST COMMON IONS:
Ir
3+
Iridium was discovered by English chemist Smithson Tenant. It was named after the Greek goddess Iris (a goddess related to the rainbow) because of the variety of color in its compounds. A very rare element (0.0001 ppm of Earth's crust), iridium is found in the naturally occurring alloys osmiridium (approximately 50% iridium) and iridiosmium (approximately 70% iridium) in Alaska and South Africa. It is obtained by igniting ammonium chloroiridate, (NH 4 ) 3 IrCl 6 , in hydrogen atmosphere. Its ground state electronic configuration is [Xe]4p 14 5d 7 6s 2 .
Iridium is the densest of all elements and its metal is lustrous, silvery, and very hard. The metal has the face-centered cubic crystal structure. Iridium has two stable isotopes : 191 Ir (37.3%) and 193 Ir (62.7%).
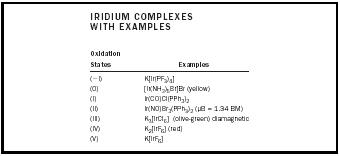
| IRIDIUM COMPLEXES WITH EXAMPLES | |
| Oxidation States | Examples |
| (−I) | K[Ir(PF 3 ) 4 ] |
| (0) | [Ir(NH 3 ) 5 Br]Br (yellow) |
| (I) | Ir(CO)Cl(PPh 3 ) 2 |
| (II) | Ir(NO)Br 3 (PPh 3 ) 2 (µB = 1.34 BM) |
| (III) | K 3 [IrCl 6 ] (olive-green) diamagnetic |
| (IV) | K 2 [IrF 6 ] (red) |
| (V) | K[IrF 6 ] |
Iridium is extremely inert to acids, but reacts slowly with oxygen and halogens at high temperatures. It forms the fluoride compounds IrF 6 , (IrF 5 ) 4 , and IrF 4 . There is some doubt of the existence of other halides with the composition IrX 4 . The most stable halides are the trihalides. Compounds that have the composition [IrX 6 ] 2− are strongly colored.
Ir 2 O 3 · n H 2 O (a brown solid) is obtained via the addition of alkali to IrCl 6 2− in the presence of H 2 O 2 . This hydrated oxide is oxidized in air to IrO 2 · n H 2 O. IrO 2 (black), with rutile structure, is the product of the reaction of Ir with O 2 at approximately 1,100°C (2,012°F); it dissociates at higher temperatures (>1,100°C, or >2,012°F).
Iridium may form complexes in its several oxidation states. Table 1 contains some examples of these complexes.
Iridium oxide, IrO 2 , is used in the fabrication of thin films for stable electrochromic materials and as an electrode material. Iridium metal is used in the manufacture of fountain pen points, airplane spark plugs, and hypodermic needles.
SEE ALSO Inorganic Chemistry .
Lea B. Zinner
Bibliography
Allred, A. L. (1961). Journal of Inorganic Nuclear Chemistry 17:215.
Cotton, F. Albert, and Wilkinson, Geoffrey (1972). Advanced Inorganic Chemistry: A Comprehensive Text, 3rd edition. New York: Interscience.
Greenwood, Norman N., and Earnshaw, A. (1984). Chemistry of the Elements. Oxford, U.K.: Pergamon.
Livingstone, Stanley E. (1973). "The Platinum Metals." In Comprehensive Inorganic Chemistry, Vol. 3, edited by J. C. Bailar Jr., et al. Oxford, U.K.: Pergamon.
Comment about this article, ask questions, or add new information about this topic: