TERBIUM

Overview
Terbium is classified as a rare earth element. The term is misleading because terbium is more common than metals such as silver and mercury. The name "rare earth" meant something else to early chemists. It was used because the rare earth elements were very difficult to separate from each other. They were not "rare" in the Earth, but they were "rarely" used for anything.
Today, chemists have developed new ways of separating elements from each other. Terbium doesn't have a great many uses, but is easily available. One use is in television screens. It helps the screen display the colors more clearly.
Although the term "rare earth" is still used, the more proper name for terbium and its cousins is the lanthanides. The term lanthanide comes from the element lanthanum in Row 6 of the periodic table. The periodic table is a chart that shows how chemcial elements are related to one another.
SYMBOL
Tb
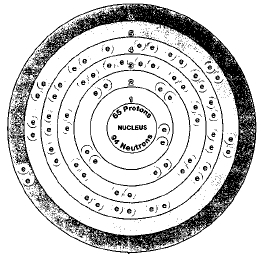
ATOMIC NUMBER
65
ATOMIC MASS
158.9254
FAMILY
Lanthanide
(rare earth metal)
PRONUNCIATION
TER-bee-um
Discovery and naming
Terbium was discovered during the great element hunt of the 1840s. That hunt began with a lucky discovery made in 1787. A lieutenant in the Swedish army, Carl Axel Arrhenius (1757-1824), discovered an unusual black rock near the town of Ytterby, Sweden. Arrhenius passed the rock on to a friend of his, chemist Johan Gadolin (1760-1852). He analyzed the sample to see what elements it contained.
Gadolin first discovered an entirely new mineral that he named yttria, after the town of Ytterby. This discovery, however, was only the first in a long chain of puzzling new findings.
In 1843, Swedish chemist Carl Gustav Mosander (1797-1858) demonstrated that yttria was really a mixture of three other minerals. He called those minerals erbia, terbia, and yttria. All three of these names also came from the town of Ytterby. The ending -a on these names means they refer to minerals that occur in the earth. A mineral ending in -a usually refers to an element combined with oxygen . For example, soda is a combination of sodium and oxygen.
Mosander's research is long and complicated. Chemists did not have good equipment in the 1840s. They often made errors and were confused by their discoveries. For example, other chemists also studied the mineral yttria. When they did so, however, they got the names Mosander used mixed up. They called his terbia "erbia" and his erbia "terbia."
Mosander is given credit for discovering terbium even though he never saw the pure element. In 1886, French chemist Jean-Charles-Galissard de Marignac (1817-94) was the first to prepare pure terbium.
Physical properties
Terbium has the silver-gray luster typical of many metals. It is quite soft, however, and can be cut with a knife. It is also malleable and ductile, meaning it can be hammered into thin sheets and drawn into wires rather easily. The melting point of terbium is 1,356°C (2,473°F) and its boiling point is about 2,800°C (5,000°F). It has a density of 8.332 grams per cubic centimeter.
Chemical properties
Like many of its rare earth cousins, terbium is not very active. It does not react with oxygen in the air very easily. It does react with water slowly, however, and will dissolve in acids.
Occurrence in nature
Terbium is one of the rarest of the Lanthanides. It ranks about 55th among the elements in the Earth's crust. It is about as abundant as molybdenum and tungsten, but more abundant than iodine, silver, and gold. Terbium occurs with other Lanthanides in minerals such as monazite, cerite, gadolinite, xenotime, and euxenite.
Isotopes
Only one isotope of terbium occurs in nature, terbium-159. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Many radioactive isotopes of terbium are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive. The 17 radioactive isotopes of terbium carry mass numbers of 147 through 158 and 160 through 164.
One radioactive isotope, terbium-149, is used in medicine. The isotope is injected directly into cancer cells in a patient's body. The radiation given off kills the cancer cells. Terbium-149 is used because its radiation does not travel far, so it does not damage healthy cells. Therefore, it is safer to use than some other radioactive isotopes.
Extraction
The rare earth elements often occur together in the earth. A mineral like monazite may contain half a dozen or more different rare earth elements. The chemist's job, then, is to find a way to separate all these elements from each other.
Today, a standard procedure is available for separating the rare earth
elements from each other. In this procedure, terbium usually ends up in
the form of the compound terbium fluoride (TbF
3
). The terbium may be obtained by passing an electric current through the
compound:
Reacting calcium metal with terbium fluoride also produces free terbium:
Uses and compounds
Probably the most common use of terbium and its compounds is in phosphors. A phosphor is a material that gives off light when struck by electrons. The back of a television screen is coated with different kinds of phosphors. When those phosphors are struck by electrons inside the television tube, they give off different colors of light. Phosphors that contain terbium give off a green light when struck by electrons. They are also used in X-ray screens to make very clear pictures.
Another use of terbium is in the manufacture of fuel cells. Any system that uses chemical reactions to produce electricity is a fuel cell. Fuel cells will probably be much more widely used as a source of electricity in the future. Terbium fuel cells operate effectively at very high temperatures.
Health effects
There is almost no information available on the health effects of terbium. In such cases, chemists use caution. The safest policy is to assume that terbium is very toxic and to avoid contact with it as much as possible.
I hope This Helps!