SILICON
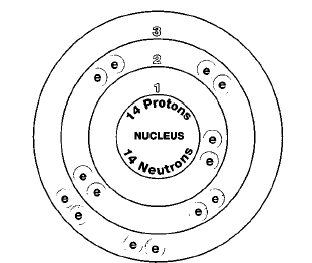
Overview
Silicon is a member of Group 14 (IVA) in the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. Silicon is also part of the the carbon family. Other carbon family elements include carbon, germanium, tin, and lead. Silicon is a metalloid, one of only a very few elements that have characteristics of both metals and non-metals.
Silicon is the second most abundant element in the Earth's crust, exceeded only by oxygen. Many rocks and minerals contain silicon. Examples include sand, quartz, clays, flint, amethyst, opal, mica, feldspar, garnet, tourmaline, asbestos, talc, zircon, emerald, and aquamarine. Silicon never occurs as a free element. It is always combined with one or more other elements as a compound.
SYMBOL
Si
ATOMIC NUMBER
14
ATOMIC MASS
28.0855
FAMILY
Group 14 (IVA)
Carbon
PRONUNCIATION
SIL-i-con
By the early 1800s, silicon was recognized as an element. But chemists had serious problems preparing pure silicon because it bonds (attaches) tightly to oxygen. It took chemists many years to find out how to separate silicon from oxygen. That task was finally accomplished in 1823 by Swedish chemist Jons Jakob Berzelius (1779-1848).
Silicon's most important application is in electronic equipment. Silicon is one of the best materials from which to make transistors and computer chips. The total weight of silicon used for this purpose is relatively small. Much larger amounts are used, for example, to make metal alloys. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals.
Discovery and naming
In one sense, humans have always used silicon. Nearly every naturally occurring rock or mineral contains some silicon. So when ancient peoples built clay huts or sandstone temples, they were using compounds of silicon.
But no one thought about silicon as an element until the nineteenth century. Then, a number of chemists tried to separate silicon from the other elements with which it is combined in the earth. English scientist Sir Humphry Davy (1778-1829) developed a technique for separating elements that tightly bond to each other. He melted these compounds and passed an electric current through them. The technique was successful for producing free or elemental sodium, potassium, calcium, and a number of other elements for the first time. But he failed with silicon. (See sidebar on Davy in the calcium entry in Volume 1.)
Berzelius also tried to isolate silicon using a method similar to that of
Davy's. He mixed molten (melted) potassium metal with a compound
known as potassium silicon fluoride (K
2
SiF
6
). The result was a new element—silicon.
Scottish chemist Thomas Thomson (1773-1852) suggested the name silicon, based on the Latin word for "flint," silex (or silids). He added the ending -on because the new element was so much like boron and carbon. Thus, the new element's name was accepted as silicon.
Some interesting studies were done on silicon over the next few years. German chemist Friedrich Wohler (1800-82) produced a series of compounds known as silanes. These compounds contain silicon, hydrogen, and, sometimes, other elements.
A group of compounds known as the siloxanes were produced at about the same time. The siloxanes are made up of silicon, oxygen, and an organic group. Organic compounds contain carbon.
Silanes and siloxanes were not discovered in the search for the answer to any practical question. Chemists were just curious about the kinds of compounds they could make with silicon. But many years later, chemists made some interesting discoveries. Both groups of compounds do have some very important practical uses. For example, the compounds known as silicones are a form of the siloxanes.
Physical properties
Silicon is a metalloid, an element with properties of both metals and non-metals. Silicon exists in two allotropic forms. Allotropes are forms of an element with different physical and chemical properties. One allotrope is in the form of shiny, grayish-black, needle-like crystals, or flat plates. The second allotrope has no crystal structure and usually occurs as a brown powder.
The melting point of silicon is 1,410°C (2,570°F) and the boiling point is 2,355°F (4,270°F). Its density is 2.33 grams per cubic centimeter. Silicon has a hardness of about 7 on the Mohs scale. The Mohs scale is a way of expressing the hardness of a material. It runs from 0 (for talc) to 10 (for diamond).
Silicon is a semiconductor. A semiconductor is a substance that conducts an electric current better than a non-conductor—like glass or rubber—but not as well as a conductor—like copper or aluminum. Semiconductors have important applications in the electronics industry.
Chemical properties
Silicon is a relatively inactive element at room temperature. It does not combine with oxygen or most other elements. Water, steam, and most acids have very little affect on the element. At higher temperatures, however, silicon becomes much more reactive. In the molten (melted) state, for example, it combines with oxygen, nitrogen, sulfur, phosphorus, and other elements. It also forms a number of alloys very easily in the molten state.
Occurrence in nature
Silicon is the second must abundant element in the Earth's crust. Its abundance is estimated to be about 27.6 percent of the crust. It ranks second only to oxygen. Some authorities believe that more than 97 percent of the crust is made of rocks that contain compounds of silicon and oxygen.
Silicon has been detected in the Sun and stars. It also occurs in certain types of meteorites known as aerolites or "stony meteorites." Meteorites are rock-like chunks that fall to the Earth's surface from outside the Earth's atmosphere.
Silicon never occurs as a free element in nature. It always occurs as a compound with oxygen, magnesium, calcium, phosphorus, or other elements. The most common minerals are those that contain silicon dioxide in one form or another. These are known as silicates.
Silicon has been detected in the Sun and stars. It also occurs in certain types of meteorites.
Isotopes
There are three naturally occurring isotopes of silicon: silicon-28, silicon-29, and silicon-30. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Five radioactive isotopes of silicon are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
None of the radioactive isotopes of silicon has any commercial use.
Extraction
Silicon is prepared by heating silicon dioxide with carbon. Carbon
replaces the silicon in the compound. The silicon formed is 96 to 98
percent pure.
Many applications of silicon require a very pure product. Methods have been developed to produce silicon that is at least 99.97 percent pure silicon. This form of silicon is called hyper-pure silicon.
Uses
Perhaps the best known use of silicon is in electronic devices. Hyperpure silicon is used in transistors and other components of electronic devices. It is also used to make photovoltaic (solar) cells, rectifiers, and parts for computer circuits. A photovoltaic cell is a device that converts sunlight into electrical energy. A rectifier is an electrical device for changing one kind of electric current (alternating current, or AC) into another kind of electric current (direct current, or DC).
Almost without exception, all glass contains silicon dioxide.
The largest single use of silicon, however, is in making alloys. The most important silicon alloys are those made with iron and steel, aluminum, and copper. When silicon is produced, in fact, scrap iron and metal is sometimes added to the furnace. As soon as the silicon is produced, it reacts with iron and steel to form ferrosilicon. Ferrosilicon is an alloy of iron or steel and silicon. It is used for two major purposes. First, it can be
The aluminum industry uses large amounts of silicon in alloys. These alloys are used to make molds and in the process of welding. Welding is a process by which two metals are joined to each other. Alloys of silicon, aluminum, and magnesium are very resistant to corrosion (rusting). They are often used in the construction of large buildings, bridges, and transportation vehicles such as ships and trains.
Compounds
A number of silicon compounds have important uses. Silicon dioxide (sand) is used in the manufacture of glass, ceramics, abrasives, as a food additive, in water filtration systems, as an insulating material, in cosmetics and Pharmaceuticals (drugs), and in the manufacture of paper, rubber, and insecticides. Each

Another important compound is silicon carbide (SiC). Silicon carbide is also known as carborundum. It is one of the hardest substances known, with a hardness of about 9.5 on the Mohs scale. Carborundum is widely used as an abrasive, a powdery material used to grind or polish other materials. Carborundum also has refractory properties. A refractory material can withstand very high temperatures by reflecting heat. Refractory materials are used to line the inside of ovens used to maintain very high temperatures.
Another important silicon group is the silicones. The silicones have an amazing range of uses. These include toys (Silly Putty and Superballs), lubricants, weatherproof!ng materials, adhesives (glues), foaming agents, brake fluids, cosmetics, polishing agents, electrical insulation, materials to reduce vibration, shields for sensitive equipment, surgical implants, and parts for automobile engines.
Health effects
Information on the health effects of silicon is limited. Some studies show that silicon may be needed in very small amounts by plants and some animals. One study showed, for example, that chickens that did not receive silicon in their diet developed minor health problems. Overall, silicon probably has no positive or negative effects on human health.
However, a serious health problem called silicosis is associated with silicon dioxide (SiO 2 ). Silicon dioxide occurs in many forms in the earth. Ordinary sand is nearly pure silicon dioxide.
In some industries, sand is ground up into a very fine powder that gets into the air. As workers inhale the dust, it travels through their mouths, down their throats, and into their lungs. Silicon dioxide powder can block the tiny air passages in the lungs through which oxygen and carbon dioxide pass. When this happens, silicosis results.
Silicosis is similar to pneumonia. The person finds it difficult to breathe. The longer one is exposed to silicon dioxide dust, the worst the problem gets. In the worst cases, silicosis results in death because of the inability to breathe properly.