NICKEL

Overview
Nickel is the only element named after the devil. The name comes from the German word Kupfernickel, meaning "Old Nick's copper," a term used by German miners. They tried to remove copper from an ore that looked like copper ore, but they were unsuccessful. Instead of copper, they got slag, a useless mass of earthy material. The miners believed the devil ("Old Nick") was playing a trick on them. So they called the fake copper ore Old Nick's copper.
Since then, nickel has become a very valuable metal. The most common use is in the production of stainless steel, a strong material that does not rust easily. It is used in hundreds of industrial and consumer applications. Nickel is also used in the manufacture of many other alloys. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals.
SYMBOL
Ni
ATOMIC NUMBER
28
ATOMIC MASS
58.69
FAMILY
Group 10 (VIIIB)
Transition metal
PRONUNCIATION
NI-kul
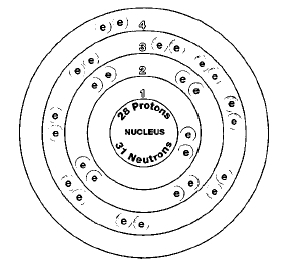
Nickel is classified as a transition metal. Transition metals are elements between Groups 2 (metals) and 13 (non-metals) in the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. Nickel is closely related to iron, cobalt, copper, and zinc. These metals are close to nickel in the periodic table.
Discovery and naming
The study of metals was difficult for early chemists. Many metals looked very similar. They also acted very much like each other chemically. Nickel was one of the metals about which there was much confusion.
Copper miners were confused about nickel and copper because they both occurred in ores with a green tint. But copper ores reacted differently to heat than did nickel ores. This confusion led to the choice for nickel's name.
But cobalt miners were confused too. Some ores of nickel also look like cobalt ores. But these ores did not react chemically in the same way either. Cobalt mine owners called the "misbehaving" ores of nickel "cobalt which had lost its soul."
Swedish mineralogist Axel Fredrik Cronstedt (1722-65) was the first person to realize that nickel was a new element. In 1751, he was given a new mineral from a cobalt mine near the town of Hälgsingland, Sweden. While Cronstedt thought the ore might contain cobalt or copper, his tests produced a surprising result. He found something in the mineral that did not act like cobalt, copper, or any other known element. Cronstedt announced that he had found a new element. He used a shortened version of Kupfernickel for the name of the new element. He called it nickel.
Physical properties
Nickel is a silvery-white metal. It has the shiny surface common to most metals and is both ductile and malleable. Ductile means capable of being drawn into thin wires. Malleable means capable of being hammered into thin sheets. Its melting point is 1,555°C (2,831°F) and its boiling point is about 2,835°C (5,135°F). The density of nickel is 8.90 grams per cubic centimeter.
Nickel is only one of three naturally occurring elements that is strongly magnetic. The other two are iron and cobalt. But nickel is less magnetic than either iron or cobalt.
Chemical properties
Nickel is a relatively unreactive element. At room temperature, it does not combine with oxygen or water or dissolve in most

It also reacts with steam to give nickel oxide and hydrogen gas:
Occurrence in nature
Nickel makes up about 0.01 to 0.02 percent of the Earth's crust. It ranks about 22nd among the chemical elements in terms of abundance in the Earth's crust. Nickel is thought to be much more abundant in the Earth's core. In fact, many experts believe that the core consists almost entirely of iron and nickel.
One argument for this belief is the presence of nickel in meteorites. Meteorites are pieces of rock or metal from space that fall to the Earth's surface. Meteorites often contain a high percentage of nickel.
The most common ores of nickel include pentlandite, pyrrhotite, and garnierite. The element also occurs as an impurity in ores of iron, copper, cobalt, and other metals.
The United States' only nickel mine is located in Riddle, Oregon. In 1996, the mine produced 15,070 tons of nickel. By comparison, Russia produced 230,000 tons of nickel in the same year. Russia is the world's largest producer of nickel Other major nickel producers are Canada (183,059 tons in 1996), New Caledonia (142,200 tons), Australia (113,134 tons), and Indonesia (90,000 tons).
The largest single deposit of nickel is located at Sudbury Basin, Ontario, Canada. The deposit was discovered in 1883. It covers an area 27 kilometers (17 miles) wide and 59 kilometers (37 miles) long. Some experts believe the deposit was created when a meteorite struck the earth at Sudbury Basin.
Isotopes
There are five naturally occurring isotopes of nickel: nickel-58, nickel-60, nickel-61, nickel-62, and nickel-64. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Seven radioactive isotopes of nickel are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
One radioactive isotope of nickel has limited use in industry, nickel-63. This isotope has two uses: for the detection of explosives, and in certain kinds of electronic devices, such as surge protectors. A surge protector is a device that protects sensitive electronic equipment like computers from sudden changes in the electric current flowing into them.
Extraction
The method used for making pure nickel metal is a common one in
metallurgy. Metallurgy is the art and science of working with metals. Most
nickel ores contain nickel sulfide (NiS). These ores are
"roasted" (heated in air). Roasting converts the nickel
sulfide to nickel oxide:

The nickel oxide is then treated with a chemical that will remove the
oxygen from the nickel. For example:
A large amount of nickel is now recycled from scrap metal. Scrap metal comes from old cars, demolition of buildings, appliances like washing machines and stoves, and landfills. The task in recycling scrap metal is to find a way to separate
Uses
The most important use of nickel is in making alloys. About 80 percent of all nickel produced in the United States in 1996 was used to make alloys. About two-thirds of that amount went into stainless steel. Stainless steel is common to household appliances (like coffee makers, toasters, and pots and pans), kitchen sink tops and stoves, and medical equipment (X-ray machines, for example). It is also used to make heavy machinery and large containers in which large-scale chemical reactions are carried out. Artists sometimes use stainless steel in sculpture because it does not rust easily. Stainless steel is important to the food and beverage, petroleum, chemical, pharmaceutical (drug), pulp and paper, and textile industries.
Nickel is also used to make the superalloys used in jet engine parts and gas turbines. Superalloys are made primarily of iron, cobalt, or nickel. They also include small amounts of other metals, such as chromium, tungsten, aluminum, and titanium. Superalloys are resistant to corrosion (rusting) and retain their properties at high temperatures.
Nickel is also very popular in the manufacture of batteries. Nickel-cadmium (nicad) and nickel-metal hydride batteries are the most popular of these batteries. They are used in a great variety of appliances, including hand-held power tools, compact disc players, pocket recorders, camcorders, cordless and cellular telephones, scanner radios, and laptop computers.
Nickel is also used in electroplating, a process by which a thin layer of one metal is laid down on top of a second metal.
Electroplating with nickel
N ickel is commonly used in electroplating. Electroplating is the process by which a thin layer of one metal is laid down on top of a second metal. Here is how electroplating is done.
First, the nickel compound to be laid down is dissolved in water. The solution may be nickel chloride (NiCl 2 ), nickel nitrate (Ni(NO 3 ) 2 ), or some other nickel compound.
Second, a sheet of the metal to be electroplated is placed into the solution. Suppose the metal is steel. The steel sheet is suspended in the nickel chloride, nickel nitrate, or other nickel solution.
Third, an electric current is passed through the solution. The current causes nickel to come out of the solution. The nickel is then deposited on the surface of the steel. The longer the current runs, the more nickel is laid down. The thickness of the nickel layer can be controlled by the time the electric current runs through the solution.
Electroplating is used to make metal products with very specific qualities. Steel is strong but tends to corrode easily. Nickel does not corrode as fast as steel. A thin layer of nickel on top of steel protects the steel from corrosion.
Compounds
Some nickel compounds have important uses also. Many of these compounds are used in electroplating. Some are used to make alloys of nickel. Other nickel compounds are used as coloring agents. For example, the compound nickel dimethylglyoxime (C 8 H 14 N 4 NiO 4 ) is used as a coloring agent in paints, cosmetics, and certain kinds of plastics.
Other nickel compounds have somewhat more unusual uses. For example, the compound nickel dibutyldithiocarbamate (Ni[CS 2 N(C 4 H 4 ) 2 ] 2 ) is used as an antioxidant in tires. The rubber in tires reacts with oxygen in the air. When it does so, the rubber gets hard and stiff. The tires begin to break down. An additive like nickel dibutyldithiocarbamate can reduce the rate at which this process occurs. The life of tires is extended.
Health effects
Nickel can pose a health hazard to certain individuals. The most common health problem is called nickel allergy. Some people are more likely to develop nickel allergy than are others. People who are sensitive to nickel may develop a skin rash somewhat like poison ivy. The rash becomes itchy and may form watery blisters. Once a person gets nickel allergy, it remains with him or her forever.
Nickel is present in dozens of products. So it is easy for sensitive people to develop nickel allergy. Perhaps the most common cause of nickel allergy is body piercing. Some people have their ears pierced for earrings, while others have their lips, nose, or other body parts pierced. Inexpensive jewelry placed into these piercings is frequently made of stainless steel. Stainless steel contains nickel. The presence of nickel in a piercing can cause nickel allergy to develop.
Nickel can cause more serious health problems too. For example, people who are exposed to nickel fumes (dust and gas) breathe in nickel on a regular basis. Long term nickel exposure may cause serious health problems, including cancer.