Robert Robinson

ENGLISH ORGANIC CHEMIST
1886–1975
An acknowledged giant of twentieth-century organic chemistry, Robert Robinson authored 700 research papers that continue to influence the way organic chemists think about synthesis , natural products, and reaction mechanisms. He received many awards during his sixty-year career, including the 1947 Nobel Prize in chemistry "for his investigations on plant products of biological importance, especially the alkaloids."
Robinson was born on September 13, 1886, near Chesterfield, England. His father owned a surgical dressing factory and invented many of the machines used to produce and package such dressings. In high school Robinson excelled in mathematics and physics and hoped to become a mathematician. However, his father encouraged him to study chemistry instead, so Robinson accepted the inevitable and entered the chemistry program at the University of Manchester.
Robinson received his D.Sc. from Manchester in 1910 and lectured there for two additional years. He then accepted successive academic appointments at Sydney, Liverpool, Manchester, London, and finally Oxford University.
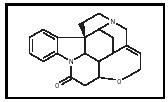
Today chemists use computer-driven instruments to determine the structures of unknown organic compounds. In Robinson's era, however, chemists relied less on instruments and more on degrading the compound into smaller, less complex fragments and then piecing them back together again. Using these techniques, Robinson determined the structures of complex alkaloids and worked on the antibiotic penicillin during World War II. His work on the structure of strychnine (see Figure 1) is still regarded as an outstanding example of molecular puzzle solving.
After structure comes synthesis, and modern chemists synthesize complex medicines and other important compounds using ideas originated by Robinson. But organic synthesis was in its infancy when Robinson started out, and in his stunning synthesis of tropinone (a compound related to cocaine) in 1917, he introduced a novel strategy for preparing complex organic compounds. On paper, Robinson disconnected, or broke, certain bonds in tropinone and arrived at three simpler building blocks. He then went to the laboratory, where he combined the three building blocks using
standard procedures and produced tropinone. This process is now called retrograde synthesis.
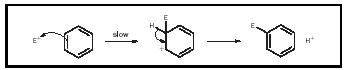
Because Robinson wanted to take a systematic approach to organic synthesis, he developed a set of theoretical tools to predict the outcomes of organic reactions. Many important drugs and natural products contain substituted benzene rings, so Robinson began his research by trying to predict the outcomes of substitution reactions in benzene derivatives. He and his wife, Gertrude, successfully explained one class of substitution reactions in a 1917 paper but were unable to provide a general theory.
Using ideas developed in Arthur Lapworth's 1922 paper, Robinson devised a new theory in 1924 that explained the chemistry of unsaturated systems such as benzene and 1,4-butadiene (a four-carbon chain with alternating double bonds). Using his new theory, Robinson successfully predicted the outcomes of chemical reactions in these unsaturated systems. And for the first time ever, he used curly arrows to show the distribution of electrons in conjugated systems and to predict substitution reactions in benzene analogs. Hardly a day goes by when a modern organic chemist does not use curly arrows to explain a reaction mechanism or to plan a synthetic route.
Although Robinson considered the curly arrow concept his most important contribution to knowledge, few chemists know he invented it. Most chemists attribute the discovery to Christopher Ingold. Ingold embraced Robinson's ideas and over time published so many of his own related papers that chemists tended to overlook Robinson's groundbreaking work. Robinson never forgave Ingold for taking credit for his ideas.
Robinson retired from Oxford in 1955 but remained active in the field of chemistry. In his younger days he climbed the Alps, Pyrenees, and major mountains in New Zealand and Norway. Chess was another of his passions: Robinson spent three years as president of the British Chess Federation. He died on February 8, 1975.
SEE ALSO Organic Chemistry .
Thomas M. Zydowsky
Bibliography
James, Laylin K., ed. (1993). Nobel Laureates in Chemistry 1901–1992. Washington, DC: American Chemical Society; Chemical Heritage Foundation.
Internet Resources
"The Nobel Prize in Chemistry 1947." Nobel e-Museum. Available from http://www.nobel.se/chemistry/laureates .
"Robert Robinson—Biography." Nobel e-Museum. Available from http://www.nobel.se/chemistry/laureates .