International System of Units
The International System of Units (SI), which began as the decimal metric system during the French Revolution, deals with the definitions, terminology, proper usage, and modifications of scientific units. The metric system was established officially in France on June 22, 1799, and consisted of two standard measures: the meter for length and the kilogram for mass. The German mathematician and astronomer Carl Friedrich Gauss (1777–1855) promoted the use of the metric system and in 1832 added the second as the unit of time. The British Association for the Advancement of Science (BAAS) in 1874 introduced an alternative system, known as the CGS system, whose units of measure were the centimeter, gram, and second. Until 1889 the scientific community had two metric standards for length, mass, and time.
The first General Conference on Weights and Measures (Conférence Générale des Poids et Mesures, or CGPM) in 1889 sanctioned a new system, the MKS system, that included the international prototypes for the meter and kilogram and the astronomical second as the unit of time. Fifty years later, in 1939, the International Committee for Weights and Measures (Comité International des Poids et Mesures, or CIPM), under authority of the CGPM, proposed a four-unit MKS system with the addition of the ampere for electric current. Official recognition of the ampere had to wait until 1946, after World War II had ended.
The tenth CGPM in 1954 added two more standards when it officially approved both the kelvin for thermodynamic temperature and the candela for luminous intensity. In 1960 the eleventh CGPM renamed its MKS system of units the International System of Units, and in 1971 the fourteenth CGPM completed the seven-unit system in use today, with the addition of the mole as the unit for the amount of a substance, setting it equal to the gram-molecular weight of a substance.
SI units fall into two groups: basic units and derived units. The basic units are the seven mutually independent units (see Table 1) and include the meter, kilogram, second, ampere, kelvin, mole, and candela. They represent,
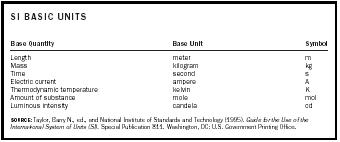
| SI BASIC UNITS | ||
| Base Quantity | Base Unit | Symbol |
| SOURCE:Taylor, Barry N., ed., and National Institute of Standards and Technology (1995). Guide for the Use of the International System of Units (SI). Special Publication 811. Washington, DC: U.S. Government Printing Office. | ||
| Length | meter | m |
| Mass | kilogram | kg |
| Time | second | s |
| Electric current | ampere | A |
| Thermodynamic temperature | kelvin | K |
| Amount of substance | mole | mol |
| Luminous intensity | candela | cd |
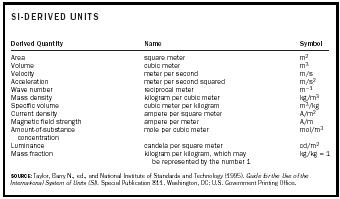
| SI-DERIVED UNITS | ||
| Derived Quantity | Name | Symbol |
| SOURCE:Taylor, Barry N., ed., and National Institute of Standards and Technology (1995). Guide for the Use of the International System of Units (SI) . Special Publication 811. Washington, DC: U.S. Government Printing Office. | ||
| Area | square meter | m 2 |
| Volume | cubic meter | m 3 |
| Velocity | meter per second | m/s |
| Acceleration | meter per second squared | m/s 2 |
| Wave number | reciprocal meter | m −1 |
| Mass density | kilogram per cubic meter | kg/m 3 |
| Specific volume | cubic meter per kilogram | m 3 /kg |
| Current density | ampere per square meter | A/m 2 |
| Magnetic field strength | ampere per meter | A/m |
| Amount-of-substance concentration | mole per cubic meter | mol/m 3 |
| Luminance | candela per square meter | cd/m 2 |
| Mass fraction | kilogram per kilogram, which may be represented by the number 1 | kg/kg = 1 |
respectively, length, mass, time, electric current, thermodynamic temperature, amount of substance, and luminous intensity. Derived units, as the name indicates, are units obtained algebraically from the seven basic units (see Table 2).
The CIPM has approved twenty prefixes for SI units (see Table 3) and permits the use of any SI prefix with an SI unit, with one exception. The SI unit for mass, the kilogram, already has a prefix in its name and can have no other SI prefix. To use prefixes with a unit for mass, the rule is to remove the "kilo" prefix and add the new prefix to "gram" (unit symbol g), as in milligram and its abbreviation mg.
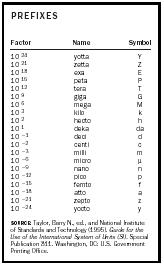
| PREFIXES | ||
| Factor | Name | Symbol |
| SOURCE:Taylor, Barry N., ed., and National Institute of Standards and Technology (1995). Guide for the Use of the International System of Units (SI). Special Publication 811. Washington, DC: U.S. Government Printing Office. | ||
| 10 24 | yotta | Y |
| 10 21 | zetta | Z |
| 10 18 | exa | E |
| 10 15 | peta | P |
| 10 12 | tera | T |
| 10 9 | giga | G |
| 10 6 | mega | M |
| 10 3 | kilo | k |
| 10 2 | hecto | h |
| 10 1 | deka | da |
| 10 −1 | deci | d |
| 10 −2 | centi | c |
| 10 −3 | milli | m |
| 10 −6 | micro | µ |
| 10 −9 | nano | n |
| 10 −12 | pico | p |
| 10 −15 | femto | f |
| 10 −18 | atto | a |
| 10 −21 | zepto | z |
| 10 −24 | yocto | y |
The CIPM has developed several rules to ensure the proper use of SI units and to eliminate ambiguity from scientific communications. The National Institute of Standards and Technology in Washington, D.C., makes available a complete detailed list of the rules.
SI units, or those the CIPM recognizes, express quantity values. Other units, if used, may appear in parentheses after the appropriate SI or recognized units. The CIPM uses no abbreviations for names and only accepted unit symbols, unit names, prefix symbols, and prefix names. It makes no differentiation in symbol use for a plural, and the only time a period follows a unit symbol is at the end of a sentence. The addition of subscripts does not change a unit name or symbol.
Mathematical operations have specific rules for the use of mathematical symbols with SI units. A space or a half-high dot represents the multiplication of units; a negative exponent, horizontal line, or slash represents the division of units, and if these mathematical symbols appear in the same line, parentheses must differentiate them. The percent sign (%) denotes the number 0.01 or 1/100, so that 1% 0.01, 30% 0.30, and so forth. Arabic numerals with the appropriate SI or recognized unit indicate the values of quantities. Commas are not used to separate numbers into groups of three. If more than four digits appear on either side of the decimal point, a space separates the groups of three.
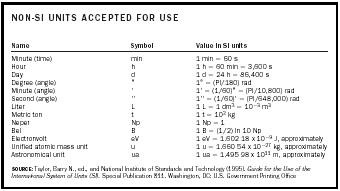
| NON-SI UNITS ACCEPTED FOR USE | ||
| Name | Symbol | Value in SI units |
| SOURCE:Taylor, Barry N., ed., and National Institute of Standards and Technology (1995). Guide for the Use of the International System of Units (SI). Special Publication 811. Washington, DC: U.S. Government Printing Office | ||
| Minute (time) | min | 1 min = 60 s |
| Hour | h | 1 h = 60 min = 3,600 s |
| Day | d | 1 d = 24 h = 86,400 s |
| Degree (angle) | ˚ | 1 ˚ = (PI/180) rad |
| Minute (angle) | ′ | 1′ = (1/60)˚ = (PI/10,800) rad |
| Second (angle) | ″ | 1″ = (1/60)′ = (PI/648,000) rad |
| Liter | L | 1L = 1 dm 3 =10 −3 m 3 |
| Metric ton | t | 1t = 10 3 kg |
| Neper | Np | 1 Np = 1 |
| Bel | B | 1B = (1/2) ln 10 Np |
| Electronvolt | eV | 1 eV = 1.602 18 × 10 −9 J, approximately |
| Unified atomic mass unit | u | 1u = 1.660 54 × 10 −27 kg, approximately |
| Astronomical unit | ua | 1 ua = 1.495 98 × 10 11 m, approximately |
Numbers, unit symbols, and names have set rules for mixing and differentiation for clarity of text and mathematical operations. These include a space between a numerical value and its unit symbol, indicating clearly the number a symbol belongs to in a given mathematical calculation, and no mixing of unit symbols and names nor making calculations on unit names. Different symbols represent values and units and the unit symbol should follow the value symbol separated by a slash. SI requires the use of standardized mathematical symbols and the explicit writing of a quotient quantity.
SI units and their symbols have distinctive type styles. Items given in italic type are variables, quantity symbols, superscripts and subscripts if they represent variables, quantities, or running numbers. Items given in roman type are unit symbols, superscripts, and subscripts that are descriptive. The typeface used in the surrounding text of the document does not change these rules.
Several terms used in vernacular science are not appropriate for scientific communication. The CIPM does not use such terms as parts per million, parts per billion, or parts per trillion or their abbreviations as expressions of quantities. The word "weight" is a force with the SI unit of newton, not a synonym for mass with the SI unit of kilogram. Terms for an object and quantities describing the object require a clear different action. Normality, molarity, and molal are obsolete terms no longer used.
Several important and widely used units are not officially part of the SI, but the CIPM has accepted them for use with SI units (see Table 4). They include the liter, day, hour, minute, electronvolt, and degree. Although the CIPM adopted the liter in 1879, it is not a current SI unit. Its symbol (L in the United States, l everywhere else) causes some confusion, but the CIPM has approved neither.
In the United States, the National Institute of Standards and Technology (NIST) also has compiled a list of units outside the SI that it has approved for domestic use (see Table 5). It recommends defining them in terms of accepted SI units. The CIPM does not encourage the use of units on the NIST list but accepts all of them, excluding the curie, roentgen, rad,
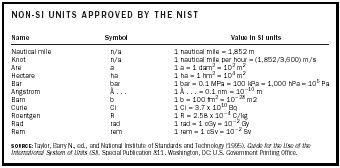
| NON-SI UNITS APPROVED BY THE NIST | ||
| Name | Symbol | Value in SI units |
| SOURCE:Taylor, Barry N., ed., and National Institute of Standards and Technology (1995). Guide for the Use of the International System of Units (SI). Special Publication 811. Washington, DC: U.S. Government Printing Office. | ||
| Nautical mile | n/a | 1 nautical mile = 1,852 m |
| Knot | n/a | 1 nautical mile per hour = (1,852/3,600) m/s |
| Are | a | 1 a = 1 dam 2 = 10 2 m 2 |
| Hectare | ha | 1 ha = 1 hm 2 = 10 4 m 2 |
| Bar | bar | 1 bar = 0.1 MPa = 100 kPa = 1,000 hPa = 10 5 Pa |
| Angstrom | Å… | 1 Å…= 0.1 nm = 10 −10 m |
| Barn | b | 1 b = 100 fm 2 = 10 −28 m2 |
| Curie | Ci | 1 Ci = 3.7 × 10 10 Bq |
| Roentgen | R | 1 R = 2.58 × 10 −4 C/kg |
| Rad | rad | 1 rad = 1 cGy = 10 −2 Gy |
| Rem | rem | 1 rem = 1 cSv = 10 −2 Sv |
and rem, which American scientists have nonetheless continued to use.
Bibliography
American Society for Testing and Materials (1993). Standard Practice for Use of the International System of Units (SI): The Modernized Metric System. E 380–93. Philadelphia: American Society for Testing and Materials.
Institute of Electrical and Electronics Engineers (1992). American National Standard for Metric Practice. ANSI/IEEE Std 266–1992. New York: Institute of Electrical and Electronics Engineers.
Mills, Ian; Cvitaš, T.; Homann, K.; Kallay, N.; and Kuchitsu, K. (1993). Quantities, Units and Symbols in Physical Chemistry, 2nd edition. Boston: Blackwell Scientific Publications.
Taylor, Barry N., ed., and National Institute of Standards and Technology (1995). Guide for the Use of the International System of Units (SI). Special Publication 811. Washington, DC: U.S. Government Printing Office.
Taylor, Barry N., ed. (1998). Interpretation of the International System of Units for the United States. Federal Register, July 28, 1998, 63, 144:40334–40340. Washington, DC: U.S. Government Printing Office.
Taylor, Barry N., ed.; National Institute of Standards and Technology (2001). The International System of Units (SI). Special Publication 330. Washington, DC: U.S. Government Printing Office.
Internet Resources
Bureau International des Poids et Mesures. "Welcome" (home page, English). Available from http://www.bipm.fr/enus/welcome.html .
National Institute of Standards and Technology, Physics Laboratory. "International System of Units." Available from http://physics.nist.gov/cuu/Units/index.html .
Comment about this article, ask questions, or add new information about this topic: