Michel Chevreul
FRENCH CHEMIST
1786–1889

Michel-Eugène Chevreul was a chemist whose career spanned the greater part of the nineteenth century. He was born in Angers, France, on August 31, 1786, and died in Paris on April 9, 1889. Chevreul's father was a wellknown physician. Raised in the midst of the terror of the French Revolution, Chevreul witnessed much violence and suffering. As a result, he maintained a lifelong aversion to politics and at an early age decided to devote his life to chemistry.
Chevreul's career as a scientist began at age seventeen when he became an assistant in the laboratory of Louis-Nicolas Vauquelin at the Muséum National d'Histoire Naturelle in Paris. While working in Vauquelin's laboratory, Chevreul began his study of organic chemistry with the investigation of the separation of natural coloring agents from their sources. At the age of twenty-four, he was named assistant naturalist at the museum. Chevreul then served as director of dyeing at the Manufacture Royale des Gobelins from 1824 to 1885. He became a member of the Academie des Sciences in 1826 and its president in 1839 and 1867. When Vauquelin retired, Chevreul attained the chair of chemistry at the museum, a position he held until his death in 1889.
Chevreul's career exemplifies the enormous strides made in the understanding of chemistry during the nineteenth century. He established the melting point as a key criterion for the purity of a substance. Even in the contemporary world, the melting point remains the first property determined to characterize a new solid. Chevreul excelled at the elemental analysis of organic substances and established the molecular formulas for many important chemical compounds. This procedure was considered very difficult in his time. His mentor, Vauquelin, had demonstrated that all foods are of four types: fats ( lipids ), proteins, starches, and sugars. In his continued studies of fats, Chevreul used the above procedures, along with various methods of purification for substances with animal origins, to identify several of the fatty acids as pure substances with consistent molecular formulas.
Chevreul also contributed to the improvement of the ancient art of soap making. He identified soaps as the potassium salts of the fatty oleic and
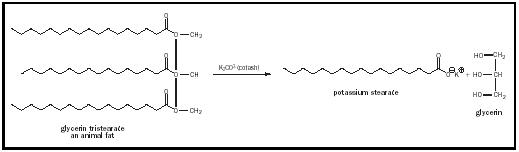
stearic acids and animal fats from which soaps were derived as esters of the alcohol glycerol. This led to the replacement of potassium carbonate ( potash ) in soap manufacture with the cheaper and more reactive sodium hydroxide (lye), which became available commercially in the early 1800s. The reaction for the manufacture of soap, saponification, is shown in Figure 1.
SEE ALSO Fats and Fatty Acids ; Soap .
Lawrence H. Brannigan
Bibliography
Aftalion, Otto (1991). A History of the International Chemical Industry , Theodor Benfey. Philadelphia: University of Pennsylvania Press.
Farber, Eduard (1952). The Evolution of Chemistry: A History of Its Ideas, Methods, and Materials. New York: Ronald Press.
Internet Resources
Cyberlipid Center. Available from http://www.cyberlipid.org .
"Michel-Eugène Chevreul." Catholic Encyclopedia. Available from http://www.newadvent.org/cathen/03650b.htm .
Comment about this article, ask questions, or add new information about this topic: