Antoine Lavoisier
FRENCH CHEMIST 1743–1794
Antoine-Laurent Lavoisier, born in Paris, France, is considered the father of modern chemistry. During the course of his career, Lavoisier managed to transform just about every aspect of chemistry. But Lavoisier was not just a scientist. He was involved in French taxation politics during a turbulent time in the country's history—the French Revolution (the first major social revolution proclaiming the liberty of the individual [ca. 1789–1799]). Because of his involvement with the ruling class, he was executed during the revolutionary days known as the Terror, at the height of his scientific career.
Just before and during the French Revolution, another revolution was taking place. In any study of the history of chemistry, the period between 1770 and 1790 is commonly regarded as the "Chemical Revolution." This revolution, which marked the beginnings of modern chemistry, occurred in large part as a result of Lavoisier's scientific excellence and brilliant experimental capabilities. He played a role in many aspects of the Chemical Revolution, including the abandonment of the phlogiston theory of combustion , the evolution of the concept of an element, and the development of a new chemical nomenclature.
Perhaps Lavoisier's most important accomplishment was his role in the dismantling of the phlogiston theory of combustion. Phlogiston was a substance believed to be emitted during combustion and the calcination of metals . Earlier chemists, such as the Germans Johann Becher (1635–1682) and George Stahl (1660–1734), supposed that a metal was composed of calx and phlogiston, and that burning resulted from the loss of phlogiston. The fact that metals actually gained weight during combustion was usually explained away by the theory that phlogiston had negative weight. Lavoisier, like some others, saw that it was illogical for anything to have negative weight.

To prove his supposition that phlogiston did not exist, Lavoisier introduced quantitative measurement to the laboratory. Using precise weighing, he showed that in all cases of combustion where an increase in weight was observed, air was absorbed, and that when a calx was burned with charcoal, air was liberated. In addition to showing by precise measurement that phlogiston did not exist, Lavoisier's findings also implied that the total weight of the substances taking part in a chemical reaction remains the same before and after the reaction—an early statement of the law of conservation of mass. By ridding the chemical world of the phlogiston theory of combustion using quantitative analysis, Lavoisier was able to push chemistry toward its modern state. No longer would counterintuitive notions such as a substance having negative weight occupy the minds of chemists.
Likewise, Lavoisier's work was also able to refute the theory that the world was composed of either one, two, three, or four elements. Lavoisier defined an element as the "last point which analysis is capable of reaching," or in modern terms, a substance that cannot be broken down any further into its components. This break from the theories of the ancient world allowed chemists to pursue the study of chemistry with a different outlook of the world. By defining elements as the last points of analysis, Lavoisier opened up new investigative possibilities. In his classic textbook Elements of Chemistry (generally acknowledged to be the first modern chemistry textbook), he compiled a list of all the substances he could not break down into simpler substances, that is, he created the first table of elements (although not the Periodic Table of later years). By acknowledging that there could be more elements than his preliminary list provided, Lavoisier left the search for more elements to his successors.
Lavoisier's dismantling of the phlogiston theory and his systematic definition of an element caused many chemists to view basic concepts differently and to embrace the principles of Lavoisier's new chemistry. One of the methods Lavoisier used to spread his ideas was to construct a new and logical system for naming chemicals. Working with Claude Berthollet and Antoine Fourcroy, Lavoisier developed a new nomenclature based on three general principles: (1) Substances should have one fixed name, (2) names ought to reflect composition when known, and (3) names should generally be chosen from Greek or Latin roots. This new nomenclature was published in 1787, and it swayed even more chemists to adopt the new chemistry.
Nevertheless, Lavoisier did not always hit on the right theories for the right reasons. For example, he believed that acidity was caused by the presence of oxygen in a compound. Lavoisier concluded in 1776 that oxygen was the part of a compound that was responsible for the property of acidity because he had isolated it from so many acids. In fact, oxygen means "acid former." According to Lavoisier, the other portion of the compound combined with the oxygen was called an "acidifiable base" and it was responsible for the specific properties of the compound. Although these concepts turned out to be wrong, the thinking behind them is important since it represented the first systematic attempt to chemically characterize acids and bases.
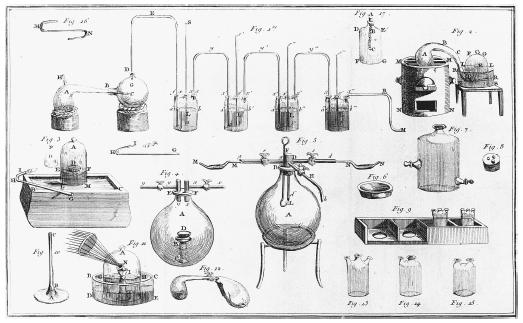
Lavoisier was not only interested in the theoretical aspects of chemistry. He also devoted much of his time to studying more practical topics, such as the best ways of lighting streets in a large town. In addition, Lavoisier took part in the development of what was to become the metric system and he was involved in improving the manufacture of gunpowder.
Although Lavoisier was independently wealthy, thanks to a considerable fortune inherited from his mother, he sought to increase his wealth in order to pursue his scientific career on a larger scale. For this reason, he entered the Ferme, a private company whose members purchased the privilege of collecting national taxes. During the French Revolution, the tax collectors of the Ferme were the subject of popular hatred. Although he carried out his duties honestly, Lavoisier was associated with the perceived corruption of the tax collection system. At the height of the Revolution, Lavoisier was arrested and executed by beheading in 1794.
Lavoisier's untimely death ended an era in the history of chemistry. With his contributions to chemistry ranging from developing the modern concept of combustion to establishing the language of chemistry, Lavoisier provided the foundation for the study of chemistry as a modern science.
SEE ALSO Berthollet, Claude-Louis .
Lydia S. Scratch
Bibliography
Donovan, Arthur (1993). Antoine Lavoisier: Science, Administration and Revolution. Oxford, U.K.: Blackwell.
Jaffe, Bernard (1976). Crucibles: The Story of Chemistry from Ancient Alchemy to Nuclear Fission. New York: Dover.
Yount, Lisa (1997). Antoine Lavoisier: Founder of Modern Chemistry. Springfield, NJ: Enslow Publishers.
Internet Resources
Beretta, Marco, ed. "Panopticon Lavoisier." Available from http://moro.imss.fi.it/lavoisier .
Poirier, Jean-Pierre. "Lavoisier's Friends." Available from http://historyofscience.free.fr .
Comment about this article, ask questions, or add new information about this topic: