RUTHENIUM

Overview
Ruthenium belongs in the platinum group of metals. The elements in this group are named after the best known member of the group, platinum. The group is found in the middle of the periodic table, in Groups 8, 9, and 10, and Rows 5 and 6. The periodic table is a chart that shows how chemical elements are related to one another. The platinum metals tend to be somewhat rare and valuable. They are also called precious metals. The platinum metals also tend to have bright, shiny surfaces and high melting points, boiling points, and densities.
Credit for the discovery of ruthenium is often given to Polish chemist Jedrzej Sniadecki (1768-1838). Sniadecki announced the discovery of the element in 1808. He suggested the name vestium for the element, after the asteroid Vesta. Other chemists were not able to confirm Sniadecki's work, however. As a result, the element was rediscovered twice more in later years.
The primary uses of ruthenium are in alloys and as catalysts for industrial processes.
SYMBOL
Ru
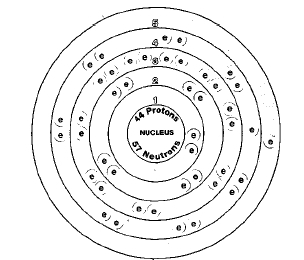
ATOMIC NUMBER
44
ATOMIC MASS
101.07
FAMILY
Group 8 (VIIIB)
Transition metal;
platinum group
PRONUNCIATION
roo-THEE-nee-um
Discovery and naming
Sniadecki discovered element 44 in 1808 while working with platinum ores from South America. After he published his results, other chemists tried to find the element as well. They were unsuccessful. Sniadecki became discouraged, dropped his claims of discovery, and did no further research on the element.
About twenty years later, the discovery of element 44 was announced again. This time, the discoverer was Russian chemist Gottfried W. Osann. Once more, other chemists could not repeat Osann's results. There was disagreement as to whether the element had been found.
Finally, in 1844, Russian chemist Carl Ernst Claus (also known in Russian as Karl Karlovich Klaus; 1796-1864) gave positive proof of a new element in platinum ores. Many authorities now call Claus the discoverer of the element. Claus suggested calling the element ruthenium, after the ancient name of Russia, Ruthenia. Osann had suggested that name as well.
Physical properties
Ruthenium is a hard, silvery-white metal with a shiny surface. Its melting point is about 2,300 to 2,450°C (4,200 to 4,400°F) and its boiling point is about 3,900 to 4,150°C (7,100 to 7,500°F). Its density is 12.41 grams per cubic centimeter.
Chemical properties
Ruthenium metal is relatively unreactive. It does not dissolve in most acids or in aqua regia. Aqua regia is a mixture of hydrochloric and nitric acids. It often reacts with materials that do not react with either acid separately. Ruthenium does not react with oxygen at room temperatures either. At higher temperatures, however, it does combine with oxygen.
Occurrence in nature
Ruthenium is one of the rarest elements in the Earth's crust. Its abundance is estimated at about 0.0004 parts per million. This makes it one of six least abundant elements on Earth.
Like other members of the platinum family, ruthenium occurs in platinum ores. It is obtained from those ores and from the mineral osmiridium by purification of the natural material.
Isotopes
Seven isotopes of ruthenium are known. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
At least nine radioactive isotopes of ruthenium are also known. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
Ruthenium-106 is used for medical purposes. When ruthenium-106 breaks down, it gives off a form of radiation called beta rays. These beta rays act somewhat like X rays. They attack and kill cancer cells. As an example, ruthenium-106 has been used to treat certain forms of eye cancer.
Extraction
Ruthenium is obtained by separating it from other platinum metals, such as platinum, palladium, and osmium, with which it occurs. These metals are usually obtained as by-products during the refining of nickel metal. They are then separated from each other by a series of chemical reactions.
Uses
One important use of ruthenium is in the manufacture of alloys. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals. Ruthenium adds two properties to an alloy. First, it makes the alloy hard. Second, it makes the alloy resistant to attack by oxygen and other materials.
Ruthenium is most often combined with platinum or palladium in alloys. Electrical contacts, devices for measuring very high and very low temperatures, and medical instruments are often made from ruthenium alloys. Some types of jewelry are also made from ruthenium, though it is very expensive.
"Ruthenium" comes from the ancient name of Russia, Ruthenia.
Ruthenium can also be alloyed with other metals. It is sometimes added to titanium to make that metal more resistant to corrosion (rusting). Only 0.1 percent of ruthenium in titanium makes titanium a hundred times more corrosion resistant.

A second important use of ruthenium is in catalysts. A catalyst is a substance used to speed up or slow down a chemical reaction without undergoing any change itself. Ruthenium catalysts may provide a way of changing light energy into electrical energy. The process is similar to photosynthesis, in which green plants change sunlight into stored chemical energy.
Compounds
No compounds of ruthenium have any important commercial application.
Health effects
Ruthenium tetroxide (RuO 4 ) is very dangerous. Its fumes are irritating to the skin, eyes, and respiratory tract (mouth, throat, and lungs).