RADON
Overview
Radon is the last member of the noble gas family. The noble gases are the elements that make up Group 18 (VIIIA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. The noble gases get their name because they are inactive chemically. They combine with other substances under only extreme conditions. Their tendency to avoid contact with other elements was seen by early chemists as "royal" or "noble" behavior. The noble gases are also called the inert gases.
Radon is a radioactive element. A radioactive element is one that gives off radiation and breaks down to form a different element. Radon is formed when heavier radioactive elements, like uranium and thorium, break down. In turn, radon breaks down to form lighter elements, such as lead and bismuth.
SYMBOL
Rn
ATOMIC NUMBER
86
ATOMIC MASS
222.0176
FAMILY
Group 18 (VIIIA)
Noble gas
PRONUNCIATION
RAY-don
Radon is a well-know air pollutant today. It is formed in rocks and soil where uranium is present. As a gas, radon tends to drift upward out of the ground. If a house or building has been built above soil containing uranium, radon may collect in the structure. The U.S. Environmental Protection Agency (EPA) regards the presence of radon in homes and offices as a serious health problem.
Discovery and naming
Radioactivity was discovered in 1896 by French physicist Antoine-Henri Becquerel (1852-1908). Becquerel observed that a photographic plate was exposed even in the dark when placed next to an ore called pitchblende. The explanation for this phenomenon was offered two years later by a colleague of Becquerel's, Polish-French chemist Marie Curie (1867-1934). Curie said that something in the pitchblende was giving off radiation. The radiation was similar to light in some ways. But it was also different, since it could not be seen. Curie suggested the name of radioactivity for this behavior.
Over the next decade, many scientists worked to find out more about radioactive materials. Curie and her husband, Pierre Curie (1859-1906), isolated two new radioactive elements, polonium and radium. In 1900, German physicist Friedrich Ernst Dorn (1848-1916) found a third radioactive element.
Dorn found this element because of an observation made by Curie. When radium is exposed to air, the air becomes radioactive. The Curies did not study this phenomenon further. However, Dorn did. Eventually he discovered that radium produces a gas when it breaks apart. The radioactive gas escapes into the air. The radioactivity of air exposed to radium is caused by this gas.
At first, Dorn called this radioactive gas radium "emanation". The term emanation refers to something that has been given off. Radium emanation, then, means something given off by radium. Dorn also considered the name of niton for the gas. This name comes from the Latin word nitens, which means "shining." Eventually, however, scientists decided on the modern name of radon. The name is a reminder of the source from which the gas comes, radium.
The proper location of radon in the periodic table was determined by Scottish chemist Sir William Ramsay (1852-1916). Ramsay was also involved in the discovery of three other noble gases, neon, krypton, and xenon. In 1903, Ramsay was able to determine the atomic weight of radon. He showed that it belonged beneath xenon in Group 18 (VIIIA) of the periodic table.
Credit for the discovery of radon is often given to other scientists as well. In 1899, Robert B. Owens announced the presence of a radioactive gas that he named thoron. In 1903, French chemist Andre Louis Debierne (1874-1949) made a similar discovery. He named the gas actinon. Certainly, some credit for the discovery of element 86 can be shared among all these men.
Physical properties
Radon is a colorless, odorless gas with a boiling point of -61.8°C (-79.2°F) . Its density is 9.72 grams per liter, making it about seven times as dense as air. It is the densest gas known. Radon dissolves in water and becomes a clear, colorless liquid below its boiling point. At even lower temperature, liquid radon freezes. As a solid, its color changes from yellow to orangish-red as the temperature is lowered even further. It is a dramatic sight since it also glows because of the intense radiation being produced.
Chemical properties
Radon was long thought to be chemically inert. The term inert means incapable of reacting with other substances. In the early 1960s, however, a number of chemists found ways of making compounds of the noble gases. They did so by combining a noble gas with a very active element. The element generally used was fluorine, the most active chemical element. The result was the formation of noble gas compounds. The first radon compound to be produced was radon fluoride (RnF).
Radon: the secret visitor
A dangerous stranger may be hiding in your home. You won't be able to see, smell, or hear the stranger. But it has the ability to cause cancer. That dangerous stranger is radon gas.
Radon is produced naturally when uranium breaks down. Uranium is a radioactive element that occurs naturally in the Earth's crust. It is a fairly common element and could be in the ground below your own home.
When uranium breaks down, it produces many different elements, including radium, thorium, bismuth, and lead. Hone of these elements is a threat since they all remain in the ground. But uranium also forms radon when it breaks down. And radon is a gas. It can float upward, out of the earth, and into the basement of your home.
In some respects, radon is a serious health hazard. It gives off radiation that can kill cells. But radon does not have a very long half life. It breaks down and disappears fairly quickly.
The problem is that it breaks down into elements that are solid. These include polonium-214, polonium-218, and lead-214. These elements are more of a threat to your health. If you inhale them, they may stick to the lining of your lungs. While there, they give off radiation. The radiation can kill or damage cells. The final result of radon escaping into a building can be a variety of respiratory problems. Respiratory problems are those affecting the lungs and other parts of the system used for breathing. The most serious of these respiratory problems is lung cancer.
Scientists today think that radon may cause as many as 20,000 cases of lung cancer per year. If so, that would make radon the second leading cause of this disease, after smoking. The people most in danger from radon are those who also smoke. These people are threatened both by radon and by cigarette smoke.
The EPA has studied the problem of radon in homes and offices. The agency believes that up to 8 million homes may have levels of radon that are too high. About 20 percent of all homes the agency has studied have high radon levels.
Fortunately, it's easy to find out if radon is lurking in your home. Radon test kits can be purchased easily and at low cost. Anyone can learn how to use one in a few minutes. If radon is present, some simple steps can be taken to reduce the danger the element presents. For example, any cracks in the foundation of a house can be sealed. By doing so, radon gas will be prevented from seeping into the house. Also, some method for circulating air should always be available. A fan or an air conditioner, for example, will insure that fresh air is constantly brought into a house and "stale" air (containing radon gas) is removed.
Occurrence in nature
The abundance of radon in air is too small to be estimated. Some radon is always present because it is formed during the breakdown of uranium and radium.
Isotopes
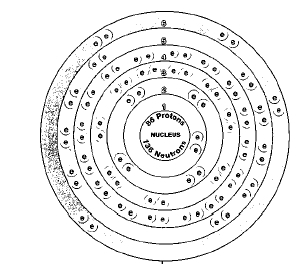
Three isotopes of radon occur in nature—radon-219, radon-220, and radon-222. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope. At least 18 other radioactive isotopes of radon have been produced artificially.
All isotopes of radon have short half-lives and do not remain in the atmosphere very long. The half life of a radioactive element or isotope is the time it takes for half of a sample of the element or isotope to break down. The radon isotope with the longest half life is radon-222 at only 2.8 days. If 10 grams of radon-222 were prepared today, only 5 grams would remain 2.8 days from now. After another 2.8 days, only 2.5 grams would be left. Within a month, it would be difficult to detect any of the isotope.
Extraction
Radon is produced during the breakdown of radium. It is obtained commercially by the following method. A compound of radium is placed under water. Gases given off by the radium compound are collected in a glass tube. Oxygen, nitrogen, water vapor, carbon dioxide, and other gases are removed from the gas in the tube. The gas that remains is pure radon.
Uses
The uses for radon all depend on the radiation it gives off. That radiation cannot be seen, smelled, tasted, or detected by any other human sense. However, a number of instruments have been invented for detecting this radiation. For example, a Geiger counter is a device that makes a clicking sound or flashes a light when radiation passes through it.
As a solid, radon changes its color from yellow to orangish-red as its temperature decreases. It is a dramatic sight since it also glows because of the intense radiation being produced.
One use of radon based on this principle is in leak detection. An isotope of radon is added to a flow of gas or liquid through
Radon was once commonly used to treat cancer too. The radiation it gives off kills cancer cells. However, the element must be used with great care because radiation can kill healthy cells as well. In fact, the bad side-effects of radiation therapy are caused by the killing of healthy cells by radiation. Today, radon is not as widely used for the treatment of cancer. More efficient isotopes have been found that are easier and safer to work with.
Compounds
Chemists are trying to make compounds of radon, but the task is difficult. One compound that has been made is radon fluoride. In any event, such compounds are laboratory curiosities and have no commercial uses.
Health effects
Because of the radiation it produces, radon is a highly dangerous material. It is used only with great caution. Radon is especially dangerous because it is inhaled, exposing fragile tissues to penetrating radiation.
thanks.