OSMIUM

Overview
Osmium is an element in Group 8 (VIIIB) of the periodic table. The periodic table is a chart showing how chemical elements are related to one another. Osmium is also a member of the platinum family. This family consists of five other elements: ruthenium, rhodium, palladium, indium, and platinum. These elements often occur together in the Earth's crust. They also have similar physical and chemical properties, and are used in alloys.
Osmium was discovered in 1804 by English chemist Smithson Tennant (1761-1815). Tennant found the new element in an ore of platinum.
Osmium is a very rare element and has few commercial uses. Osmium tetroxide (OsO 4 ), is more widely used, however, because it is so active.
SYMBOL
Os
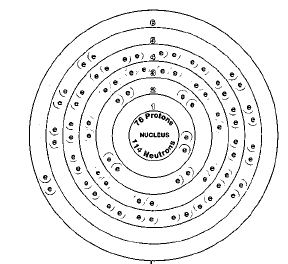
ATOMIC NUMBER
76
ATOMIC MASS
190.2
FAMILY
Group 8 (VIIIB)
Transition metal
Platinum group
PRONUNCIATION
OZ-mee-um
Discovery and naming
Platinum metal (atomic number 78) was known to chemists as early as 1741. Over the next 60 years, however, scientists discovered that the substance they knew as "platinum" was usually a mixture of substances. These substances proved to be new elements. Osmium was one of the new elements discovered in impure platinum.
In the early 1800s, Smithson Tennant was studying platinum. He found that a black powder remained when platinum was dissolved in aqua regia. Aqua regia is a mixture of hydrochloric and nitric acids. The term aqua regia means "royal water." It often dissolves materials that either add by itself does not dissolve.
In 1804, Tennant announced that the black powder was actually a mixture of two new elements. He called them indium and osmium. He suggested osmium's name because of the unusual smell of the compound he was working with, osmium tetroxide. Osmium comes from the Greek word osme, meaning "odor."
Physical properties
Osmium is a bluish-white, shiny metal with a melting point of about 3,000°C (5,400°F) and a boiling point of about 5,500°C (9,900°F). Its density is 22.5 grams per cubic centimeter. These numbers are the highest of any platinum metal. They are also among the highest of all elements.
Osmium is unworkable as a metal. It cannot be melted and shaped like most metals. Because it is unworkable, it has very few practical uses.
Chemical properties
Osmium is dissolved by acids or by aqua regia only after long periods of exposure to the liquids. When heated, the metal combines with oxygen to form osmium tetroxide (OsO 4 ). Osmium tetroxide is very toxic and the only important commercial compound of osmium.
Occurrence in nature
Osmium is very rare. Its abundance is thought to be about 0.001 parts per million (one part per billion). That places the element among the half dozen least abundant elements in the Earth's crust.
The most common ore of osmium is osmiridium. The element also occurs in all ores of platinum.
Isotopes
There are seven naturally occurring isotopes of osmium. The most abundant are osmium-192, osmium-190, and osmium-189
Many radioactive isotopes of osmium are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive. No radioactive isotope of osmium has any important use.
Extraction
Osmium is obtained when platinum metal is extracted from its ores.
Uses
Osmium metal has few uses. It is sometimes added to platinum or indium to make them harder. Some of the best pen points, for example, are made of osmium-platinum alloys. An alloy is made by melting or mixing two or more metals. The osmium-platinum alloy is harder than pure platinum. Some alloys of osmium and platinum are also used to make specialized laboratory equipment.
Finely divided osmium metal is also used as a catalyst. A catalyst is a substance used to speed up or slow down a chemical reaction. The catalyst does not undergo any change itself during the reaction. The process for making ammonia from combined hydrogen and nitrogen sometimes uses osmium as a catalyst.
Compounds
Osmium tetroxide (OsO 4 ) is in demand for use as a catalyst for research purposes. The problem is that this compound of osmium is very dangerous to use. It is shipped in small glass containers called ampules. The ampules carry no labels, nor are they marked with ink. The label and ink would react violently with osmium tetroxide. Users are instructed to open and use an ampule containing osmium tetroxide with great care.
Osmium tetroxide is so dangerous to use that it is shipped in a small glass container. The container carries no label or ink because each would react violently with the compound.
Health effects
Some compounds of osmium are extremely dangerous. They irritate the respiratory passage (throat, lungs, etc.), the skin, and the eyes. They must be handled with extreme care. This caution is especially important for the most widely used compound of osmium, osmium tetroxide.