MANGANESE
Overview
Manganese is a transition metal. The transition metals are the large block of elements in the middle of the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. The transition metals make up Rows 4 through 7 in Groups 3 through 12 of the periodic table. Many of the best known and most widely used metals are in this group of elements.
It took chemists some time to discover the difference between manganese and iron. The two metals have very similar properties and often occur together in the Earth's crust. The first person to clearly identify the differences between the two elements was Swedish mineralogist Johann Gottlieb Gahn (1745-1818) in 1774.
SYMBOL
Mn
ATOMIC NUMBER
25
ATOMIC MASS
54.9380
FAMILY
Group 7 (VIIB)
Transition metal
PRONUNCIATION
MANG-guh-neez
Manganese plays an interesting role in the U.S. economy. It is absolutely essential in the production of iron and steel. No element has been found that can replace manganese is such applications. The United States has essentially no manganese supplies of its own, so it depends on imports from other nations.
Discovery and naming
One of the main ores of manganese is pyrolusite. Pyrolusite is made up primarily of the compound manganese dioxide (MnO 2 ). Early artists were familiar with pyrolusite. They used the mineral to give glass a beautiful purple color. They also used the mineral to remove color from a glass. When glass is made, it often contains impurities that give the glass an unwanted color. The presence of iron, for example, can give glass a yellowish tint. Adding pyrolusite to yellowish glass removes the color. The purple tint of pyrolusite balances out the yellow color of the glass. The glass ends up being clear and colorless.
By the mid-1700s, chemists began to suspect that pyrolusite might contain a new element. Some authorities credit German chemist Ignatius Gottfried Kaim with isolating the element in 1770. However, Kaim's report was not read by many chemists and was quickly lost.
During this period, some of the most famous chemists in Europe were trying
to analyze pyrolusite, but none of them was successful. The problem was
solved in 1774 when Gahn developed a method for removing the new element
from pyrolusite. He heated pyrolusite with charcoal (pure
carbon
). The carbon took
oxygen
away from manganese dioxide, leaving behind pure manganese:
The origin of manganese's name is a bit confusing. Early chemists associated the new element with a mineral called magnesia. That mineral got its name because it is magnetic. Magnesia does not contain manganese, but the name stuck.
Physical properties
Manganese is a steel-gray, hard, shiny, brittle metal. It is so brittle, in fact, that it cannot be machined in its pure form. Machining refers to the bending, cutting, and shaping of a metal by mechanical means. The melting point of manganese is 1,245°C (2,273°F) and its boiling point is about 2,100°C (3,800°F). Its density is 7.47 grams per cubic centimeter.
Manganese exists in four allotropic forms. Allotropes are forms of an element with different physical and chemical properties. The element changes from one form to another as the temperature rises. The form that exists from room temperature up to about 700°C (1,300°F) is the most common form.
Chemical properties
Manganese is a moderately active metal. It combines slowly with oxygen in the air to form manganese dioxide (MnO 2 ). At higher temperatures, it reacts more rapidly. It may even burn, giving off a bright white light. Manganese reacts slowly with cold water, but more rapidly with hot water or steam. It dissolves in most acids with the release of hydrogen gas. It also combines with fluorine and chloride to make manganese difluoride (MnF 2 ) and manganese dichloride (MnCl 2 ).
Occurrence in nature
Manganese never occurs as a pure element in nature. It always combines with oxygen or other elements. The most common ores of manganese are pyrolusite, manganite, psilomelane, and rhodochrosite. Manganese is also found mixed with iron ores. The largest producers of manganese ore in the world are China, South Africa, the Ukraine, Brazil, Australia, Gabon, and Kazakstan.
Manganese also occurs abundantly on the ocean floor in the form of nodules. These nodules are fairly large lumps of metallic ores. They usually contain cobalt, nickel, copper, and iron, as well as manganese. Scientists estimate that up to 1.5 trillion metric tons of manganese nodules may lie on the floors of the world's oceans and large lakes. Currently, there is no profitable method for removing these ores.
Manganese is the 12th most abundant element in the Earth's crust. Its abundance is estimated to be 0.085 to 0.10 percent. That makes it about as abundant as fluorine or phosphorus.
Up to 1.5 trillion metric tons of manganese nodules (large lumps of metallic ores) may lie on ocean floors.
Isotopes
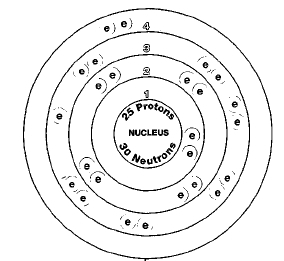
Only one naturally occurring isotope of manganese exists, manganese-22. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.

Nine radioactive isotopes of manganese are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
None of the radioactive isotopes of manganese has any important commercial uses.
Extraction
The usual method for producing pure manganese is to heat manganese dioxide
(MnO
2
) with carbon or aluminum. These elements remove the oxygen and leave pure
metal:
Uses
Up to 90 percent of all manganese produced is made into steel alloys. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals. The addition of manganese to steel makes the final product hard, as well as resistant to corrosion (rusting) and mechanical shock.
The most common alloy of manganese is ferromanganese. This alloy contains about 48 percent manganese combined with iron and carbon. Ferromanganese is the starting material for making a very large variety of steel products, including tools, heavy-duty machinery, railroad tracks, bank vaults, construction components, and automotive parts. About 60 percent of the manganese used in the United States in 1996 went to the manufacture of ferromanganese.
Another common alloy of manganese is silicomanganese. It contains manganese, silicon, and carbon in addition to iron. It is used for structural components and in springs. The production of silicomanganese accounted for about 33 percent of the manganese used in the United States in 1996.
Manganese is also used to make alloys with metals other than iron or steel. For example, the alloy known as manganin is 84 percent copper, 12 percent manganese, and 4 percent nickel. Manganin is used in electrical instruments.
Compounds
Less than 10 percent of all the manganese used in the United States goes to the production of manganese compounds. Perhaps the most important commercial use of these compounds is manganese dioxide (MnO 2 ). Manganese dioxide is used to make dry-cell batteries. These batteries are used in electronic equipment, flashlights, and pagers. Dry cell batteries hold a black pasty substance containing manganese dioxide. The use of manganese dioxide in a dry cell prevents hydrogen gas from collecting in the battery as electricity is produced.
Another manganese compound, manganous chloride (MnCl 2 ), is an additive in animal food for cows, horses, goats, and other domestic animals. Fertilizers also contain manganous chloride so that plants get all the manganese they need.
Finally, small amounts of manganese compounds are used as coloring agents in bricks, textiles, paints, inks, glass, and ceramics. Manganese compounds can be found in shades of pink, rose, red, yellow, green, purple, and brown.
Health effects
Manganese is one of the chemical elements that has both positive and negative effects on living organisms. A very small amount of the element is needed to maintain good health in plants and animals. The manganese is used by enzymes in an organism. An enzyme is a molecule that makes chemical reactions occur more quickly in cells. Enzymes are necessary to keep any cell operating properly. If manganese is missing from the diet, enzymes do not operate efficiently. Cells begin to die, and the organism becomes ill.
Fortunately, the amount of manganese needed by organisms is very small. It is not necessary to take extra manganese to meet the needs of cells.
Manganous chloride (MnCl 2 ) is an additive in animal food.
In fact, an excess of manganese can create health problems. These problems include weakness, sleepiness, tiredness, emotional disturbances, and even paralysis. The only way to receive such a large dose is in a factory or mine. Workers may inhale manganese dust in the air.