GOLD

Overview
Gold has been called the most beautiful of all chemical elements. Its beauty has made it desirable for use in jewelry, coins, and artwork for thousands of years. It was one of the first pure metals to be used by humans.
Gold is one of the few elements that can affect politics and economics. Wars have been fought over access to gold. Cities and towns have sprung up and died out as gold was discovered and then mined out. Many nations still count their wealth according to the amount of gold they keep in storage.
Gold lies in the middle of the periodic table. The periodic table is a chart that shows how elements are related to one another. Gold is a heavy metal in a group known as the transition metals. Gold is also known as a precious metal (as are platinum and silver).
Large amounts of gold are still used in the manufacture of coins, medals, jewelry, and art. Gold also has a number of uses in industry, medicine, and other applications. For example, one radioactive isotope of gold is commonly used to treat cancer.
SYMBOL
Au
ATOMIC NUMBER
79
ATOMIC MASS
196.9665
FAMILY
Group 11 (IB)
Transition metal
PRONUNCIATION
GOLD
The chemical symbol for gold is Au. The symbol comes from the Latin word for gold, aurum. Aurum means "shining dawn."
Discovery and naming
Gold objects dating to 2600 B.C. have been found. They were discovered in the royal tombs of the ancient civilization of Ur. These objects showed that humans had already learned how to work with gold this early in history. Some of the gold, for example, had been formed into wires.
One of the special skills developed by the Egyptians was the adding of gold to glass objects. They found a way to use gold to make glass a beautiful ruby-red color. The glass became known as gold ruby glass.
Gold is also mentioned in a number of places in the Bible. A passage in Exodus, for example, refers to the clothing worn by Aaron: "And they did beat the gold into thin plates, and cut it into wires, to work it in the blue, and in the purple, and in the scarlet, and in the fine linen, with cunning work."
Writings from every stage of human history tell of the discovery and use of gold. Roman historian Pliny the Elder ( A.D. 23-79), for example, describes gold-mining locations. The Romans found it lying in stream beds in the Tagus River in Spain, the Po River in Italy, the Hebrus River in Thracia (now Greece), the Pactolus River in Asia Minor (now Turkey), and the Ganges River in India.
Goin' for the silver and gold!
O ne of the most famous items using gold is the Olympic gold medal. Athletes from around the world dream of coming in first place at the Olympics. That means they can step up to the winner's podium and wear their gold medal proudly. But the gold medal isn't solid gold. It's actually made out of silver. A thin layer of gold covers the silver. The last time a solid gold medal was used in the Olympics was 1912.
Christopher Columbus found gold nuggets lying in the bottom of rivers and harbors in Haiti.
Gold has long been known in the New World, too. During a visit to Haiti, Christopher Columbus (1451-1506) found gold nuggets lying on the bottom of rivers and harbors. A Portuguese explorer in 1586, Lopez Vaz, wrote that the region called Veragua (now Panama) was the "richest Land of Gold [in] all the rest of the Indies."

In the United States, of course, the most famous story about gold occurred in the late 1840s. Thousands of people flocked to California in search of gold. This era was called the Gold Rush. People became very rich or found nothing at all during this exciting time in history.
Physical properties
Gold is both ductile and malleable. Ductile means it can be drawn into thin wires. Malleable means capable of being hammered into thin sheets. A piece of gold weighing only 20 grams (slightly less than an ounce) can be hammered into a sheet that will cover more than 6 square meters (68 square feet). The sheet will be only 0.00025 centimeters (one ten-thousandth of an inch) thick. Gold foil of this thickness is often used to make the lettering on window signs.
Gold is quite soft. It can usually be scratched by a penny. Its melting point is 1,064.76°C (1,948.57°F) and its boiling point is about 2,700°C (4,900°F). Its density is 19.3 grams per cubic centimeter.
Two other important properties are its reflectivity and lack of electrical resistance. Both heat and light reflect off gold very well. But an electric current passes through gold very easily.
Chemical properties
Generally speaking, gold is not very reactive. It does not combine with oxygen or dissolve in most acids. It does not react with halogens, such as chlorine or bromine , very easily.
These chemical properties also account for some important uses of gold. Gold coins, for example, do not corrode (rust) or tarnish very easily. Neither does jewelry or artwork made of gold.
Occurrence in nature
Gold occurs in nature in both its native state and in compounds. The native state of an element is its free state. It is not combined with any other element. The most common compounds of gold are the tellurides. A telluride is a compound of the element tellurium and one or more other elements. For example, the mineral calavarite is mostly gold telluride (AuTe 2 ).
At one time, gold was found in chunks or nuggets large enough to see. People mined gold by picking it out of streams and rivers. In fact, gold was once very common in some parts of the world. People valued it not because it was rare, but because it was so beautiful.
The abundance of gold in the Earth's crust is estimated to be about 0.005 parts per million. That makes it one of the ten rarest elements in the Earth's crust. Gold is thought to be much more common in the oceans. Some people believe as much as 70 million tons of gold are dissolved in seawater. They also think there may be another 10 billion tons on the bottom of the oceans. So far, however, no one has found a way to mine this gold.
About a quarter of the world's gold comes from South Africa. Other leading producers of the metal are the United States, Australia, Canada, China, and Russia. In the United States, about two-thirds of its gold is mined in Nevada. California, Montana, Alaska, and South Dakota also produce gold.
The Gold Rush!
T he most famous story about gold in the United States might be the Gold Rush of 1849. As early as the sixteenth century, records contained stories about a great El Dorado ("the gilded one," in Spanish; gilded means "covered in gold") on the western coast of the United States. Tales of this magical city were repeated for centuries.
In the late 1840s, explorers began to travel from the Eastern seaboard to California in search of El Dorado. The flow of visitors was slow at first. Gold was first discovered in 1848 at a place called Sutter's Mill. Sutter's Mill was located near the present town of Coloma, California.
Word of the discovery spread quickly. Within a year, thousands of men and women made the long, expensive, and tiring trip. Most people traveled across the United States in covered wagons or on horseback. Many of them had to cross mountains, plains, and deserts. Because of the difficult conditions, many people and animals got sick or died. Some people traveled around Cape Horn at the bottom of South America or across the Isthmus of Panama. No matter which route was used, the journey usually took months.
As people arrived in California, hundreds of mining camps sprang up. Some of them had colorful names. Poker Flat, Hangtown, Red Dog, Hell's Delight, and Whiskey Bar were just a few! Mining for gold was hard work. Gold miners usually wound up being wildly successful or terrible failures. The Gold Rush of 1849 completely changed the state of California. It also helped expand the United States.
At one time, gold was found in chunks or nuggets large enough to see.
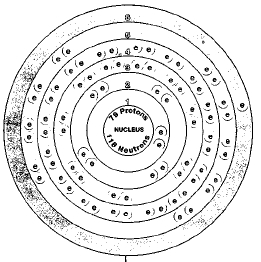
Isotopes
There is only one naturally occurring isotope of gold, gold-197. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons

About two dozen radioactive isotopes of gold are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
One radioactive isotope of gold is widely used in medicine, gold-198. This isotope has two major uses. First, it can be used to study the liver. It is made into a form known as colloidal gold. Colloidal gold consists of very fine particles of gold mixed in a liquid solution. The colloidal gold is injected into the patient's body, where it travels to the liver. There, it can be detected because of the radiation it gives off. The radiation can be used to tell if the liver is functioning normally or not.
In a 1986 study, experts estimated that 121,000 tons of gold had been mined throughout history.
Colloidal gold is also used to treat medical problems. In some forms of cancer, the body develops large amounts of liquid in the space around the stomach and intestines (the peritoneum). One way to treat this collection of liquid is with colloidal gold. The colloidal gold is injected into the peritoneum.
It is not able to Leave the peritoneum and go into the stomach and intestines. While in the peritoneum, the colloidal gold gives off radiation. The radiation kills cancer cells that cause the accumulation of fluid.
Extraction
There are at least two main ways to remove gold from its ores. One is to mix an ore with mercury metal. Mercury combines with gold in the ore to form an amalgam. An amalgam is a mixture of two or more metals, one of which is mercury. The gold amalgam is then removed from the ore. It is heated to drive off the mercury. Pure gold remains.
Measuring gold
Which weighs more: A pound of feathers or a pound of gold? Teachers sometimes try to fool students with this old question. The answer would seem to be easy: a pound is a pound. A pound of feathers and a pound of gold should weigh the same amount.
But that is not quite true. In the English system, most substances are measured using the avoirdupois (pronounced a-verde-POIZ) system. In the avoirdupois system, there are 16 ounces to the pound.
But gold is weighed differently. It uses the troy system. In the troy system, one pound contains only 12 ounces. So, a pound of feathers (avoirdupois system) weighs four ounces more than a pound of gold (troy system). The weight of other precious metals, like silver and platinum, are also measured using the troy system.
Gold is also weighed in carats. A carat is defined as one fifth of a gram, or 200 milligrams.
Gold is seldom used in a pure form. The metal is too soft. It would bend or break if used pure. Instead, it is used in combination with other metals called alloys. An alloy is a mixture of two or more metals. The mixture has properties different from those of the individual metals.
The amount of gold in an alloy is expressed in carats. Pure gold metal (mixed with no other metal) is said to be 24-carat gold. An alloy that contains 20 parts of gold and 4 parts of silver is 20-carat gold. The "20-carat" designation means the alloy contains 20 parts of gold and 4 parts of something else (silver, in this case).
Gold stored in a national bank can be 24-carat gold. It is never used for any practical purpose. But gold used for any real application is almost always less than 24 carats. It must include other metals that make it stronger and tougher.
Gold ores can also be treated with potassium cyanide (KCN) or some other kind of cyanide. The gold combines with the cyanide to form a new compound, gold cyanate. The gold cyanate is then treated with an active metal, such as zinc. The active metal replaces gold in the compound, leaving pure gold.
Uses
In a 1986 study, experts estimated that 121,000 tons of gold had been mined throughout history. Of that amount, about 18,000 tons were used for industrial, research, health, and other "dissipative" uses. Dissipative means that the gold was gone once it was used. It was made into devices that were eventually thrown away. The gold could not or was not recovered from the devices.
Of the remaining 103,000 tons of gold, about a third (35,000 tons) had been made into gold bars held by national banks. The gold bars are used as security for national money systems. In the United States, for example, the nation's supply of gold is stored at Fort Knox, Kentucky.
Finally, the remaining 68,000 tons of gold are owned by private individuals. This gold exists in the form of jewelry, coins, or bullion. Gold bullion are bars or other large pieces of pure gold.
Jewelry is the largest single use of gold. In 1996, about 3,290 tons of gold were made worldwide. Of that amount, nearly 85 percent was made into jewelry. The second largest use of gold (about 213 tons, or about 7 percent) was in industrial devices and consumer products. Some examples include electrical contacts and switches, laboratory equipment, printed circuits, dental alloys, instruments on space vehicles, and nozzles used in the production of synthetic fibers.
Compounds
Few gold compounds have any important commercial uses.
In the United States, the nation's supply of gold is stored at Fort Knox, Kentucky.
Health effects
Gold is not required to maintain good health in plants or animals. It can be injected into a plant or animal without causing harmful effects. Some medical and commercial uses are based on this property.
Thanks a lots!